Abstract
Background
Gastrointestinal stromal tumors (GIST) treatment changed considerably with introduction of imatinib in 2001 and reports of early successes. However, little is known about imatinib incorporation into practice. Our objective was to examine the integration of adjuvant systemic therapy into GIST management.
Methods
Patients with gastric GIST were identified (n = 4508) from the National Cancer Data Base (2001–2007). Separate regression models were developed to examine factors associated with adjuvant and neoadjuvant therapy use.
Results
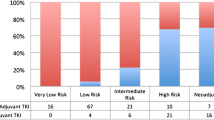
A total of 3050 patients underwent surgical resection. From 2001–2003 to 2006–2007, use of adjuvant therapy increased from 29 to 47% (P < 0.001). Patients were less likely to receive adjuvant therapy if tumors were <3 cm, low grade, had negative margins, were treated at low-volume centers, or were diagnosed during 2001–2003 (P < 0.01). Adjuvant systemic therapy for lesions <3 cm also increased (17 to 25%, P = 0.001). For high-risk GISTs, adjuvant therapy use increased from 41 to 58% overall, with increases of 46 to 70% at high-volume centers and 40 to 48% at low-volume centers (P < 0.001). Neoadjuvant therapy increased from 0 to 8%; patients were more likely to receive neoadjuvant treatment if their tumor was >6 cm, treated at high-volume centers, or were diagnosed during 2006–2007 (P < 0.001).
Conclusions
Adjuvant systemic therapy use for GISTs was increasing and widespread prior to FDA approval of adjuvant imatinib, suggesting that contemporaneous advances in management of advanced GIST were being simultaneously and rapidly translated into the adjuvant setting. As relatively costly therapies are integrated into practice, more robust tracking systems are needed to monitor the incorporation of new treatments.


Similar content being viewed by others
References
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–6.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7.
Lemonick M, Park A, Cray D, Gorman C. New hope for cancer. Time. 2001;157:62–9.
Blanke CD, von Mehren M, Joensuu H, Roberts PJ, et al. Evaluation of the safety and efficacy of an oral molecularly-targeted, STI571, in patients with unresectable or metastatic gastrointestinal stromal tumors expressing C-KIT (CD117). Proc Am Soc Clin Oncol. 2001 (abstr 1).
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80.
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–5.
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34.
DeMatteo RP, Owzar K, Antonescu CR, Maki R, Demetri D, McCarter M, et al. Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: the U.S. Intergroup phase II trial ACOSOG Z9000. Proc Am Soc Clin Oncol. 2008 (abstr 8).
Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104.
DeMatteo RP, Owzar K, Maki R, Pisters P, Blackstein M, Antonescu C, et al. Adjuvant imatinib mesylate increases recurrence free survival in patients with completely resected localized primary gastrointestinal stromal tumor: North American Intergroup Phase III trial ACOSOG Z9001. Proc Am Soc Clin Oncol. 2007 (abstract 10079).
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–7.
McAuliffe JC, Hunt KK, Lazar AJ, Choi H, Qiao W, Thall P, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16:910–9.
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Cancer Netw. 2010; 8 Suppl 2:S1–41; quiz S42–4.
Balas E, Boren S. Managing clinical knowledge for health care improvement. In: Bemmel J, Mc Cray AT, editors. Yearbook of medical informatics 2000: patient-centered systems. Stuttgart: Schattauer; 2000. p. 65–70.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90.
Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3.
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–81.
International Classification of Disease for Oncology. 3rd ed. Geneva: World Health Organization, 2000.
Facility Oncology Registry Data Standards. Chicago: Commission on Cancer, 2004.
Department of Health and Human Services. The International Classification of Diseases. 9th revised. clinical modification: ICD-9-CM. Washington, DC: Government Printing Office, 1998.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
Commission on Cancer: Approvals Categories. Available at http://www.facs.org/cancer/coc/categories.html. Accessed December 17, 2006.
Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139:658–65.
Hosmer J, Lemeshow S. Applied logistic regression. New York: Wiley, 1999.
Eckhauser F, Yahanda A, Greenson J. Gastric smooth muscle tumors. In: Cameron J, editor. Current surgical therapy, 3rd ed. St. Louis: Mosby; 1998. p. 114–7.
Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65.
Miettinen M, El-Rifai W, Sobin LH, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–83.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78.
Huse DM, von Mehren M, Lenhart G, Joensuu H, Blanke C, Feng W, et al. Cost effectiveness of imatinib mesylate in the treatment of advanced gastrointestinal stromal tumours. Clin Drug Investig. 2007;27:85–93.
Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011;37:75–88.
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–9.
Van Glabbeke M, Verweij J, Casali PG, Le Cesne A, Hohenberger P, Ray-Coquard I, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–804.
Gold JS, Dematteo RP. Neoadjuvant therapy for gastrointestinal stromal tumor (GIST): racing against resistance. Ann Surg Oncol. 2007;14:1247–8.
Bilimoria KY, Bentrem DJ, Feinglass JM, Stewart AK, Winchester DP, Talamonti MS, et al. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626–33.
Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–83.
Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80.
Bilimoria KY, Balch CM, Bentrem DJ, Talamonti MS, Ko CY, Lange JR, et al. Complete lymph node dissection for sentinel node-positive melanoma: assessment of practice patterns in the United States. Ann Surg Oncol. 2008;15:1566–76.
Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–34.
Bilimoria KY, Balch CM, Wayne JD, Chang DC, Palis BE, Dy SM, et al. Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol. 2009;27:1857–63.
Andtbacka RH, Ng CS, Scaife CL, Cormier JN, Hunt KK, Pisters PW, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007;14:14–24.
Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–300.
Cress RD, Zaslavsky AM, West DW, Wolf RE, Felter MC, Ayanian JZ. Completeness of information on adjuvant therapies for colorectal cancer in population-based cancer registries. Med Care. 2003;41:1006–12.
Du XL, Key CR, Dickie L, Darling R, Delclos GL, Waller K, et al. Information on chemotherapy and hormone therapy from tumor registry had moderate agreement with chart reviews. J Clin Epidemiol. 2006;59:53–60.
Rubin JL, Taylor DC, Sanon M, Coombs JH, Bollu VK. Budgetary impact of treatment with adjuvant imatinib for 1 year following surgical resection of Kit-positive localized gastrointestinal stromal tumors. J Manag Care Pharm. 2010;16:482–91.
Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? Milbank Q. 2005;83:843–95.
Brook RH. Assessing the appropriateness of care—its time has come. JAMA. 2009;302:997–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bilimoria, K.Y., Wayne, J.D., Merkow, R.P. et al. Incorporation of Adjuvant Therapy into the Multimodality Management of Gastrointestinal Stromal Tumors of the Stomach in the United States. Ann Surg Oncol 19, 184–191 (2012). https://doi.org/10.1245/s10434-011-1842-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1842-9