Abstract
Background
Telomerase is widely expressed in most human cancers, but is almost undetectable in normal somatic cells and is therefore a potential drug target. Using the human telomerase promoter platform, the naturally occurring compound butylidenephthalide (BP) was selected for subsequent investigation of antitumor activity in vitro and in vivo.
Methods
We treated human glioblastoma cells with BP and found a dose-dependent decrease in human telomerase reverse transcriptase (hTERT) mRNA expression and a concomitant increase in p16 and p21 expression. Because c-Myc and Sp1 are involved in transcriptional regulation of hTERT, the effect of BP on c-Myc and Sp1 expression was examined.
Results
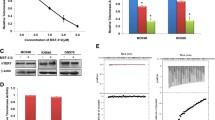
Using electrophoretic mobility shift assays and western blotting, we showed that BP represses hTERT transcriptional activity via downregulation of Sp1 expression. Using the telomerase repeat amplification protocol, an association between BP concentration and suppression of telomerase activity, induction of human glioblastoma senescence, and inhibition of cellular proliferation was identified. This was supported by a mouse xenograft model, in which BP repressed telomerase and inhibited tumor proliferation, resulting in tumor senescence. Overexpression of hTERT restored telomerase activity in human glioblastoma cells and overcame replicative senescence.
Conclusions
These findings suggest that BP inhibits proliferation and induces senescence in human glioblastomas by downregulating hTERT expression and consequently telomerase activity. This is the first study to describe regulation of telomerase activity by BP in human glioblastomas.






Similar content being viewed by others
References
Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008;1142:108–32.
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–16.
Lee HW, Blasco MA, Gottlieb GJ, Horner JW II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74.
Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–8.
von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–93.
Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–72.
Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science. 1995;269:1236–41.
Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–95.
Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–22.
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–9.
Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–4.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91.
Le S, Zhu JJ, Anthony DC, Greider CW, Black PM. Telomerase activity in human gliomas. Neurosurgery. 1998;42:1120–4; discussion 1124–5.
Ozawa T, Gryaznov SM, Hu LJ, Pongracz K, Santos RA, Bollen AW, et al. Antitumor effects of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. Neuro Oncol. 2004;6:218–26.
Cheng YL, Chang WL, Lee SC, Liu YG, Chen CJ, Lin SZ, et al. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 2003;73:2383–94.
Yim TK, Wu WK, Pak WF, Mak DH, Liang SM, Ko KM. Myocardial protection against ischaemia-reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother Res. 2000;14:195–9.
Ye YN, Koo MW, Li Y, Matsui H, Cho CH. Angelica sinensis modulates migration and proliferation of gastric epithelial cells. Life Sci. 2001;68:961–8.
Ye YN, Liu ES, Li Y, So HL, Cho CC, Sheng HP, et al. Protective effect of polysaccharides-enriched fraction from Angelica sinensis on hepatic injury. Life Sci. 2001;69:637–46.
Ye YN, Liu ES, Shin VY, Koo MW, Li Y, Wei EQ, et al. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem Pharmacol. 2001;61:1439–48.
Ye YN, So HL, Liu ES, Shin VY, Cho CH. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci. 2003;72:925–32.
Abebe W. Herbal medication: potential for adverse interactions with analgesic drugs. J Clin Pharm Ther. 2002;27:391–401.
Tsai NM, Lin SZ, Lee CC, Chen SP, Su HC, Chang WL, et al. The antitumor effects of Angelica sinensis on malignant brain tumors in vitro and in vivo. Clin Cancer Res. 2005;11:3475–84.
Tsai NM, Chen YL, Lee CC, Lin PC, Cheng YL, Chang WL, et al. The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J Neurochem. 2006;99:1251–62.
Chen YL, Jian MH, Lin CC, Kang JC, Chen SP, Lin PC, et al. The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma (HCC) cells therapy. Mol Pharmacol. 2008;74:1046–58.
Lin PC, Chen YL, Chiu SC, Yu YL, Chen SP, Chien MH, et al. Orphan nuclear receptor, Nurr-77 was a possible target gene of butylidenephthalide chemotherapy on glioblastoma multiform brain tumor. J Neurochem. 2008;106:1017–26.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5.
Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113:3613–22.
Janik P, Briand P, Hartmann NR. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975;35:3698–704.
Nakano K, Watney E, McDougall JK. Telomerase activity and expression of telomerase RNA component and telomerase catalytic subunit gene in cervical cancer. Am J Pathol. 1998;153:857–64.
Boldrini L, Faviana P, Gisfredi S, Zucconi Y, Di Quirico D, Donati V, et al. Evaluation of telomerase in the development and progression of colon cancer. Int J Mol Med. 2002;10:589–92.
Park TW, Riethdorf S, Riethdorf L, Löning T, Jänicke F. Differential telomerase activity, expression of the telomerase catalytic sub-unit and telomerase-RNA in ovarian tumors. Int J Cancer. 1999;84:426–31.
Kanaya T, Kyo S, Takakura M, Ito H, Namiki M, Inoue M. hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int J Cancer. 1998;78:539–43.
Kelland LR. Telomerase inhibitors: targeting the vulnerable end of cancer? Anticancer Drugs. 2000;11:503–13.
Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, et al. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer. 2002;97:621–5.
Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, et al. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127–36.
Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184–92.
Cookson JC, Dai F, Smith V, Heald RA, Laughton CA, Stevens MF, et al. Pharmacodynamics of the G-quadruplex-stabilizing telomerase inhibitor 3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]acridinium methosulfate (RHPS4) in vitro: activity in human tumor cells correlates with telomere length and can be enhanced, or antagonized, with cytotoxic agents. Mol Pharmacol. 2005;68:1551–8.
Gowan SM, Harrison JR, Patterson L, Valenti M, Read MA, Neidle S, et al. A G-quadruplex-interactive potent small-molecule inhibitor of telomerase exhibiting in vitro and in vivo antitumor activity. Mol Pharmacol. 2002;61:1154–62.
Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 2006;18:254–60.
Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–4.
Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 2000;28:669–77.
Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A. 1998;95:14723–8.
Jagadeesh S, Kyo S, Banerjee PP. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006;66:2107–15.
Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775:5–20.
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell. 2004;14:501–13.
Keith WN, Thomson CM, Howcroft J, Maitland NJ, Shay JW. Seeding drug discovery: integrating telomerase cancer biology and cellular senescence to uncover new therapeutic opportunities in targeting cancer stem cells. Drug Discov Today. 2007;12:611–21.
Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–7.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7.
Coates SS, Lehnert BE, Sharma S, Kindell SM, Gary RK. Beryllium induces premature senescence in human fibroblasts. J Pharmacol Exp Ther. 2007;322:70–9.
Liu JP. Telomerase: not just black and white, but shades of gray. Mol Cell Biol Res Commun. 2000;3:129–35.
Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–79.
Keith WN, Bilsland A, Evans TR, Glasspool RM. Telomerase-directed molecular therapeutics. Expert Rev Mol Med. 2002;4:1–25.
Acknowledgment
We thank Robert A. Weinberg for the pCI neo-hEST2-HA (Addgene plasmid 1782) construct. Grant: This work was supported by grants from the National Science Council of the Republic of China (96-2320-B-039-044-MY3), from the Center for Neuropsychiatry, China Medical University and Hospital, Taichung, Taiwan, and from China Medical University and Hospital, Taichung, Taiwan (DMR-99-076). This study is supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH99-TD-B-111-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
P-C. Lin and S-Z. Lin contributed equally to this work.
H-J. Harn and T-W. Chiou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2011_1644_MOESM1_ESM.eps
Electronic Supplementary Material: Supplementary Figures 1 and 2 are available for this article at doi:10.1245/s10434-011-1644-0 and are accessible for authorized users. (EPS 1430 kb)
Rights and permissions
About this article
Cite this article
Lin, PC., Lin, SZ., Chen, YL. et al. Butylidenephthalide Suppresses Human Telomerase Reverse Transcriptase (TERT) in Human Glioblastomas. Ann Surg Oncol 18, 3514–3527 (2011). https://doi.org/10.1245/s10434-011-1644-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1644-0