Abstract
Background
Outcome prediction after resection with curative intent for pancreatic ductal adenocarcinoma remains a challenge. There is increasing evidence that the presence of an ongoing systemic inflammatory response is associated with poor outcome in patients undergoing resection for a variety of common solid tumors. Our aim was to prospectively evaluate the prognostic value of tumor- and patient-related factors including the systemic inflammatory response in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma of the head of pancreas.
Methods
The prognostic impact of tumor factors such as stage and host factors, including the systemic inflammatory response (modified Glasgow Prognostic Score [mGPS]), were evaluated in a prospective study of 135 patients who underwent elective pancreaticoduodenectomy for pancreatic ductal adenocarcinoma from January 2002 to April 2009.
Results
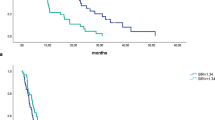
In addition to the established tumor-related pathological factors (in particular margin involvement; hazard ratio [HR] 2.82, 95% confidence interval [CI] 1.65–4.84, P < 0.001), an elevated mGPS (HR 2.26, 95% CI 1.43–3.57, P < 0.001) was independently associated with lower overall survival after pancreaticoduodenectomy. Additionally, in an adjuvant therapy subgroup of 74 patients, both margin involvement and an elevated mGPS remained independently associated with reduced overall survival.
Conclusions
We have prospectively validated the influence of tumor-related and patient-related factors. Margin involvement and the preoperative mGPS were the most important determinants of overall survival in patients undergoing potentially curative pancreaticoduodenectomy. Furthermore, both had independent prognostic value in those patients receiving adjuvant chemotherapy. In the future, this may be considered a stratification factor for entry onto therapeutic trials.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7.
Luttges J, Schemm S, Vogel I, et al. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol. 2000;191:154–61.
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79.
van Roest MH, Gouw AS, Peeters PM, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumor localization. Ann Surg. 2008;248:97–103.
Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–94.
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210.
Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251:1003–10.
Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81.
Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8.
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–6.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63.
Vashist YK, Loos J, Dedow J, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. doi:10.1245/s10434-010-1383-7.
McMillan DC, Crozier JE, Canna K, et al. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–6.
Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–51.
Jamieson NB, Glen P, McMillan DC, et al. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer. 2005;92:21–3.
Glen P, Jamieson NB, McMillan DC, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–3.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54.
Monreal M, Fernandez-Llamazares J, Pinol M, et al. Platelet count and survival in patients with colorectal cancer—a preliminary study. Thromb Haemost. 1998;79:916–8.
Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97:1266–70.
Kandemir EG, Mayadagli A, Karagoz B, et al. Prognostic significance of thrombocytosis in node-negative colon cancer. J Int Med Res. 2005;33:228–35.
Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4.
Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Smith RA, Ghaneh P, Sutton R, et al. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–8.
Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–68.
Greene FL, Page DL, Fleming ID, et al. Exocrine pancreas. In: Greene FL, Page DL, Fleming ID, et al, editors. AJCC cancer staging manual, 6th ed. New York: Springer; 2002. p. 157–64.
Royal College of Pathologists. Standards and minimum datasets for reporting cancers. minimum dataset for the histopathological reporting of pancreatic, ampulla of Vater and bile duct carcinoma. London: Royal College of Pathologists; 2002.
Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30.
Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14:999–1011.
Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–8.
Ramsey S, Lamb GW, Aitchison M, et al. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–12.
Vigano A, Bruera E, Jhangri GS, et al. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160:861–8.
Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–62.
Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–60.
Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–7.
Smith RA, Dajani K, Dodd S, et al. Preoperative resolution of jaundice following biliary stenting predicts more favourable early survival in resected pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2008;15:3138–46.
Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60.
Sewnath ME, Karsten TM, Prins MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17–27.
van der Gaag NA, Rauws EJA, van Eijick CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:130–7.
Sharma R, Zucknick M, London R, et al. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008;7:331–7.
Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–35.
Crozier JE, McKee RF, McArdle CS, et al. The presence of a systemic inflammatory response predicts poorer survival in patients receiving adjuvant 5-FU chemotherapy following potentially curative resection for colorectal cancer. Br J Cancer. 2006;94:1833–6.
Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009;250:268–72.
Mantovani G, Maccio A, Madeddu C, et al. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med. 2010;88:85–92.
Acknowledgment
N.B.J. undertook this work while supported by a Clinical PhD Fellowship from the Chief Scientist’s Office of the Scottish Government. The authors thank Dr. Ian Stewart for diagnostic imaging, Dr. Alex MacDonald for medical oncology management, pancreatic audit secretary Diane Stewart, and the West of Scotland Pancreatic Unit nurse specialists Elspeth Cowan, Alison Sinclair, and Linda Dewar for follow-up data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamieson, N.B., Denley, S.M., Logue, J. et al. A Prospective Comparison of the Prognostic Value of Tumor- and Patient-Related Factors in Patients Undergoing Potentially Curative Surgery for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 18, 2318–2328 (2011). https://doi.org/10.1245/s10434-011-1560-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1560-3