Abstract
Background
A previous study identified midkine (MK) expression in primary gastrointestinal stromal tumor (GIST) as a prognostic marker. The aim of the current study was to compare serum midkine (S-MK) concentrations of GIST patients with those of healthy controls and to determine if MK can serve as a prognostic serum marker for these patients.
Materials and Methods
S-MK concentrations were measured by enzyme-linked immunosorbent assay in GIST patients (n = 96) and healthy controls (n = 148). S-MK levels were then correlated with clinicopathological data and the administration of imatinib therapy. In addition, MK expression was evaluated in 39 surgically resected GIST and in 17 leiomyoma specimens on a tissue microarray.
Results
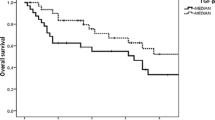
S-MK concentrations in GIST patients were significantly higher than in healthy controls: median (25th and 75th percentiles) S-MK concentration was 235 (139 and 376) pg/ml in the GIST patients and 99 (33 and 198) pg/ml in the controls (P < 0.001; Mann–Whitney U test). Significantly higher median S-MK concentrations were found in GIST with recurrence compared with those without (295 vs 230; P = 0.009). GIST patients with S-MK levels higher than 400 pg/ml showed a significantly worse recurrence-free survival (P = 0.026; log-rank test). Patients receiving imatinib therapy had decreased median S-MK concentrations compared with those who were not treated with imatinib (331 vs 201; P < 0.001).
Conclusions
S-MK concentration is a potential marker for evaluating the progression and prognosis of GIST, especially during imatinib therapy. Further studies could focus on the role of midkine in the tumorigenesis of GIST and responsiveness toward imatinib therapy.





Similar content being viewed by others
References
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81–9.
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8.
Miettinen M, El-Rifai W, L HLS, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–83.
Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39–51.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80.
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65.
Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol. 2001;54:96–102.
Schurr P, Wolter S, Kaifi JT, Reichelt U, Kleinhans H, Wachowiak R, et al. Microsatellite DNA alterations of gastrointestinal stromal tumors are predictive for outcome. Clin Cancer Res. 2006;12:5151–57.
Kadomatsu K. Recent progress of midkine research on cancer. Nippon Rinsho. 2000;58:1337–47.
Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–8.
Takei Y, Kadomatsu K, Yuasa K, Sato W, Muramatsu T. Morpholino antisense oligomer targeting human midkine: its application for cancer therapy. Int J Cancer. 2005;114:490–7.
Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, et al. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701–6.
Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–43.
Friedrich C, Holtkamp N, Cinatl J, Jr., Sakuma S, Mautner VF, Wellman S, et al. Overexpression of Midkine in malignant peripheral nerve sheath tumor cells inhibits apoptosis and increases angiogenic potency. Int J Oncol. 2005;27:1433–40.
Fiegel HC, Kaifi JT, Wachowiak R, Quaas A, Aridome K, Ichihara-Tanaka K, et al. Midkine is highly expressed in neuroblastoma tissues. Pediatr Surg Int. 2008;24:1355–9.
Ikematsu S, Nakagawara A, Nakamura Y, Sakuma S, Wakai K, Muramatsu T, et al. Correlation of elevated level of blood midkine with poor prognostic factors of human neuroblastomas. Br J Cancer. 2003;88:1522–6.
Mishima K, Asai A, Kadomatsu K, Ino Y, Nomura K, Narita Y, et al. Increased expression of midkine during the progression of human astrocytomas. Neurosci Lett. 1997;233:29–32.
Shimada H, Nabeya Y, Okazumi S, Matsubara H, Kadomatsu K, Muramatsu T, et al. Increased serum midkine concentration as a possible tumor marker in patients with superficial esophageal cancer. Oncol Rep. 2003;10:411–4.
Tanabe K, Matsumoto M, Ikematsu S, Nagase S, Hatakeyama A, Takano T, et al. Midkine and its clinical significance in endometrial carcinoma. Cancer Sci. 2008;99:1125–30.
Shimada H, Nabeya Y, Tagawa M, Okazumi S, Matsubara H, Kadomatsu K, et al. Preoperative serum midkine concentration is a prognostic marker for esophageal squamous cell carcinoma. Cancer Sci. 2003;94:628–32.
Obata Y, Kikuchi S, Lin Y, Yagyu K, Muramatsu T, Kumai H. Serum midkine concentrations and gastric cancer. Cancer Sci. 2005;96:54–6.
Kaifi JT, Fiegel HC, Rafnsdottir SL, Aridome K, Schurr PG, Reichelt U, et al. Midkine as a prognostic marker for gastrointestinal stromal tumors. J Cancer Res Clin Oncol. 2007;133:431–5.
Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res. 1999;5:1966–75.
Ota K, Fujimori H, Ueda M, Shiniriki S, Kudo M, Jono H, et al. Midkine as a prognostic biomarker in oral squamous cell carcinoma. Br J Cancer. 2008;99:655–62.
Maeda S, Shinchi H, Kurahara H, Mataki Y, Noma H, Maemura K, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97:405–11.
Ren YJ, Zhang QY. Expression of midkine and its clinical significance in esophageal squamous cell carcinoma. World J Gastroenterol. 2006;12:2006–10.
Kaifi JT, Strelow A, Schurr PG, Reichelt U, Yekebas EF, Wachowiak R, et al. L1 (CD171) is highly expressed in gastrointestinal stromal tumors. Mod Pathol. 2006;19:399–406.
Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, et al. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266–74.
Takahashi R, Tanaka S, Hiyama T, Ito M, Kitadai Y, Sumii M, et al. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797–802.
Huang Y, Cao G, Wang H, Wang Q, Hou Y. The expression and location of midkine in gastric carcinomas of Chinese patients. Cell Mol Immunol. 2007;4:135–40.
Maehara H, Kaname T, Yanagi K, Hanzawa H, Owan I, Kinjou T et al. Midkine as a novel target for antibody therapy in osteosarcoma. Biochem Biophys Res Commun. 2007;358:757–62.
Wang Q, Huang Y, Ni Y, Wang H, Hou Y. siRNA targeting midkine inhibits gastric cancer cells growth and induces apoptosis involved caspase-3,8,0 activation and mitochondrial depolarization. J Biomed Sci. 2007;14:783–95.
Acknowledgment
We thank Sabine Schmidt for her excellent technical assistance. We thank the patients who willingly and generously provided data and samples for research purposes.
Financial Support
This study was funded by research grants from the Dr. Mildred Scheel Stiftung (German Cancer Aid) and supported by a Research Grant from Novartis Oncology, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded by research grants from the Dr. Mildred Scheel Stiftung (German Cancer Aid) and supported by a Research Grant from Novartis Oncology, Switzerland.
Rights and permissions
About this article
Cite this article
Rawnaq, T., Kunkel, M., Bachmann, K. et al. Serum Midkine Correlates with Tumor Progression and Imatinib Response in Gastrointestinal Stromal Tumors. Ann Surg Oncol 18, 559–565 (2011). https://doi.org/10.1245/s10434-010-1191-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1191-0