Abstract
Background and Objectives
The p53 gene promotes cell-cycle arrest and apoptosis upon DNA damage and is associated with chemo- and radiosensitivity of cancer cells. However, its clinical significance has not been confirmed, especially in squamous cell carcinoma of the esophagus (ESCC). We investigated the correlation between p53 disorders (gene mutation and protein accumulation) and the effects of chemoradiotherapy (CRT).
Patients and Methods
Biopsy specimens obtained before CRT (40–60 Gy; low-dose 5-fluorouracil plus cisplatin) from 64 patients with locally advanced (T2–T4) ESCC were examined for p53 gene mutations (MT) of exons 4–9 by polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) and protein accumulation by immunohistochemistry (IHC). These were correlated with the pathological effects of CRT and cause-specific survival.
Results
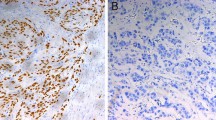
Pathological complete response (pCR) was observed in 21.9% (14/64) patients, who showed better survival than non-pCR patients (2-year survival 78.6% versus 40.5%, P = 0.007). p53 mutation (MT)+ and p53 IHC+ were observed in 31.3% (20/64) and 65.6% (42/64) patients, respectively, and each was significantly associated with non-pCR (P = 0.004 and 0.042, respectively). Combined evaluation of p53 MT and p53 IHC correlated well with pCR frequency, showing 0% (0/12) for MT+/IHC+, 0% (0/8) for MT+/IHC–, 20% (6/30) for MT–/IHC+ and 57.1% (8/14) for MT–/IHC–. These results indicate that presence of p53 mutations was associated with non-pCR regardless of IHC status, and that p53 immunoreactivity was helpful in predicting non-pCR among p53 mutation-negative patients.
Conclusion
Analysis of ESCC biopsy specimens for p53 gene mutation can identify patients who will not achieve pCR by CRT. The results should be confirmed by large cohort prospective studies.

Similar content being viewed by others
References
Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg. 1994;219(3):310–6.
Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220(3):364–72 (discussion 372–3).
Nakamura T, Hayashi K, Ota M, et al. Salvage esophagectomy after definitive chemotherapy and radiotherapy for advanced esophageal cancer. Am J Surg. 2004;188(3):261–6.
Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol. 2007;25(26):4110–7.
Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25.
al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15(1):277–84.
Adham M, Baulieux J, Mornex F, et al. Combined chemotherapy and radiotherapy followed by surgery in the treatment of patients with squamous cell carcinoma of the esophagus. Cancer. 2000;89(5):946–54.
Tepper J. Refluxtions on esophageal cancer: can we swallow the changes? J Clin Oncol. 2000;18(3):453–4.
Lane DP. Cancer p53, guardian of the genome. Nature. 1992;358(6381):15–6.
Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329(18):1318–27.
Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54(18):4855–78.
Lowe SW, Bodis S, McClatchey A, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266(5186):807–10.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74(6):957–67.
Tanaka N, Ishihara M, Kitagawa M, et al. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994;77(6):829–39.
Ribeiro U, Jr., Finkelstein SD, Safatle-Ribeiro AV, et al. p53 sequence analysis predicts treatment response and outcome of patients with esophageal carcinoma. Cancer. 1998;83(1):7–18.
Benhattar J, Cerottini JP, Saraga E, et al. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer. 1996;69(3):190–2.
Kunisaki C, Imada T, Yamada R, et al. Prognostic factors after chemoradiotherapy for patients with inoperable esophageal squamous cell carcinoma. Hepatogastroenterology. 2006;53(69):366–71.
Shimada Y, Watanabe G, Yamasaki S, et al. Histological response of cisplatin predicts patients’ survival in oesophageal cancer and p53 protein accumulation in pretreatment biopsy is associated with cisplatin sensitivity. Eur J Cancer. 2000;36(8):987–93.
Kitamura K, Saeki H, Kawaguchi H, et al. Immunohistochemical status of the p53 protein and Ki-67 antigen using biopsied specimens can predict a sensitivity to neoadjuvant therapy in patients with esophageal cancer. Hepatogastroenterology. 2000;47(32):419–23.
Wang LS, Chow KC, Chi KH, et al. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94(7):1933–40.
Kajiyama Y, Hattori K, Tomita N, et al. Histopathologic effects of neoadjuvant therapies for advanced squamous cell carcinoma of the esophagus: multivariate analysis of predictive factors and p53 overexpression. Dis Esophagus. 2002;15(1):61–6.
Casson AG, Tammemagi M, Eskandarian S, et al. p53 alterations in oesophageal cancer: association with clinicopathological features, risk factors, and survival. Mol Pathol. 1998;51(2):71–9.
Sobin LH. TNM classification of malignant tumors, 6th edition. New York: Wiley;2002.
Kolble K. The LEICA microdissection system: design and applications. J Mol Med. 2000;78(7):B24–5.
Bosari S, Marchetti A, Buttitta F, et al. Detection of p53 mutations by single-strand conformation polymorphisms (SSCP) gel electrophoresis. A comparative study of radioactive and nonradioactive silver-stained SSCP analysis. Diagn Mol Pathol. 1995;4(4):249–55.
Orita M, Iwahana H, Kanazawa H, et al. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA. 1989;86(8):2766–70.
Hattori K, Kajiyama Y, Tsurumaru M. Mutation of the p53 gene predicts lymph node metastases in Japanese patients with esophageal carcinoma: DNA and immunohistochemical analyses. Dis Esophagus. 2003;16(4):301–6.
Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, 9th ed; 1999.
Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11(6):1118–23.
Coia LR. Chemoradiation as primary management of esophageal cancer. Semin Oncol. 1994;21(4):483–92.
Popp MB, Hawley D, Reising J, et al. Improved survival in squamous esophageal cancer Preoperative chemotherapy and irradiation. Arch Surg. 1986;121(11):1330–5.
Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91(11):2165–74.
Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2009;137(1):49–54.
Zhang X, Watson DI, Jamieson GG, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma. Dis Esophagus. 2005;18(2):104–8.
Poplin E, Fleming T, Leichman L, et al. Combined therapies for squamous-cell carcinoma of the esophagus, a Southwest Oncology Group Study (SWOG-8037). J Clin Oncol. 1987;5(4):622–8.
Lackey VL, Reagan MT, Smith RA, Anderson WJ. Neoadjuvant therapy of squamous cell carcinoma of the esophagus: role of resection and benefit in partial responders. Ann Thorac Surg. 1989;48(2):218–21.
Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol. 1990;8(1):119–27.
Wolfe WG, Vaughn AL, Seigler HF, et al. Survival of patients with carcinoma of the esophagus treated with combined-modality therapy. J Thorac Cardiovasc Surg. 1993;105(4):749–55 (discussion 755–6).
Stewart JR, Hoff SJ, Johnson DH, et al. Improved survival with neoadjuvant therapy and resection for adenocarcinoma of the esophagus. Ann Surg. 1993;218(4):571–6; (discussion 576–8).
Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg. 2001;88(3):338–56.
Heath EI, Burtness BA, Heitmiller RF, et al. Phase II evaluation of preoperative chemoradiation and postoperative adjuvant chemotherapy for squamous cell and adenocarcinoma of the esophagus. J Clin Oncol. 2000;18(4):868–76.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–7.
Yang B, Rice TW, Adelstein DJ, et al. Overexpression of p53 protein associates decreased response to chemoradiotherapy in patients with esophageal carcinoma. Mod Pathol. 1999;12(3):251–6.
Uchino S, Saito T, Inomata M, et al. Prognostic significance of the p53 mutation in esophageal cancer. Jpn J Clin Oncol. 1996;26(5):287–92.
Coggi G, Bosari S, Roncalli M, et al. p53 protein accumulation and p53 gene mutation in esophageal carcinoma A molecular and immunohistochemical study with clinicopathologic correlations. Cancer. 1997;79(3):425–32.
Finlay CA, Hinds PW, Tan TH, et al. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8(2):531–9.
Cripps KJ, Purdie CA, Carder PJ, et al. A study of stabilisation of p53 protein versus point mutation in colorectal carcinoma. Oncogene. 1994;9(9):2739–43.
Bosari S, Viale G, Roncalli M, et al. p53 gene mutations, p53 protein accumulation and compartmentalization in colorectal adenocarcinoma. Am J Pathol. 1995;147(3):790–8.
Sun XF, Carstensen JM, Zhang H, et al. Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet. 1992;340(8832):1369–73.
Bosari S, Viale G, Bossi P, et al. Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J Natl Cancer Inst. 1994;86(9):681–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makino, T., Yamasaki, M., Miyata, H. et al. p53 Mutation Status Predicts Pathological Response to Chemoradiotherapy in Locally Advanced Esophageal Cancer. Ann Surg Oncol 17, 804–811 (2010). https://doi.org/10.1245/s10434-009-0786-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0786-9