Abstract
Background
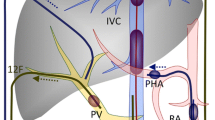
Isolated hepatic perfusion (IHP) of chemotherapy has been proposed to deliver high doses of drug while avoiding systemic toxicity. Hypotonic cisplatin has a high in vitro activity on human colon cancer cells. We studied the safety of a 30-min hypoxic single-pass IHP with hypotonic cisplatin.
Methods
A preliminary in vitro assay was performed to compare the cytotoxicity of cisplatin and oxaliplatin, in either a normotonic or hypotonic medium. Cisplatin in hypotonic medium was then chosen for the in vivo IHP. Eleven pigs underwent 30 min of IHP with 0, 50, 75, or 100 mg/L of cisplatin in a hypotonic solution under total vascular exclusion of the liver. Clinical and biological parameters were recorded for 30 days, and liver histology was performed at necropsy. The cytotoxic activity of the effluent against resistant human colon cancer cells was tested in vitro.
Results
No hepatic failure was recorded after IHP with cisplatin, but limited foci of necrosis were found at necropsy in animals receiving 75 or 100 mg/L of cisplatin. No clinical, biological, macroscopic, or microscopic toxicity was observed after IHP with 50 mg/L of hypotonic cisplatin. The liver effluent showed high in vitro cytotoxic activity against colon cancer cells.
Conclusions
A hypoxic single-pass isolated liver perfusion with hypotonic cisplatin is feasible and safe. Effluent from the liver is highly cytotoxic on cancer cells. A clinical study with 50 mg/L of hypotonic cisplatin is warranted in patients with unresectable liver metastases from colon cancer.


Similar content being viewed by others
References
Elias D, Goere D, Boige V, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol. 2007;14:3188–94.
Morise Z, Sugioka A, Kato R, et al. Transarterial chemoembolization with degradable starch microspheres, irinotecan, and mitomycin-C in patients with liver metastases. J Gastrointest Surg. 2006;10:249–58.
Noda T, Ohigashi H, Ishikawa O, et al. Liver perfusion chemotherapy for selected patients at a high-risk of liver metastasis after resection of duodenal and ampullary cancers. Ann Surg. 2007;246:799–805.
de Vries MR, Borel Rinkes IH, van de Velde CJ, et al. Isolated hepatic perfusion with tumor necrosis factor alpha and melphalan: experimental studies in pigs and phase I data from humans. Recent Results Cancer Res. 1998;147:107–19.
Hafström LR, Holmberg SB, Naredi PL. Isolated hyperthermic liver perfusion with chemotherapy for liver malignancy. Surg Oncol. 1994;3:103–8.
Lindnér P, Fjälling M, Hafström L, et al. Isolated hepatic perfusion with extracorporeal oxygenation using hyperthermia, tumour necrosis factor alpha and melphalan. Eur J Surg Oncol. 1999;25:179–85.
Vahrmeijer AL, van Dierendonck JH, Keizer HJ, et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 2000;82:1539–46.
van Iersel LB, Verlaan MR, Vahrmeijer AL, et al. Hepatic artery infusion of high-dose melphalan at reduced flow during isolated hepatic perfusion for the treatment of colorectal metastases confined to the liver: a clinical and pharmacologic evaluation. Eur J Surg Oncol. 2007;33:874–81.
Rothbarth J, Tollenaar RA, van de Velde CJ. Recent trends and future perspectives in isolated hepatic perfusion in the treatment of liver tumors. Expert Rev Anticancer Ther. 2006;6:553–65.
Taeger G, Grabellus F, Podleska LE, Müller S, Ruchholtz S. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia. 2008;24:193–203.
van Iersel LB, Gelderblom H, Vahrmeijer AL, et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 2008;19:1127–34.
van Iersel LB, Hoekman EJ, Gelderblom H, et al. Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Ann Surg Oncol. 2008;15:1891–8.
Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–87.
Alexander HR Jr, Libutti SK, Pingpank JF, et al. Isolated hepatic perfusion for the treatment of patients with colorectal cancer liver metastases after irinotecan-based therapy. Ann Surg Oncol. 2005;12:138–44.
Alexander HR Jr, Libutti SK, Bartlett DL, et al. Hepatic vascular isolation and perfusion for patients with progressive unresectable liver metastases from colorectal carcinoma refractory to previous systemic and regional chemotherapy. Cancer. 2002;95:730–6.
Rothbarth J, Pijl ME, Vahrmeijer AL, et al. Isolated hepatic perfusion with high-dose melphalan for the treatment of colorectal metastasis confined to the liver. Br J Surg. 2003;90:1391–7.
Alexander HR Jr, Bartlett DL, Libutti SK, et al. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:1852–9.
Zeh HJ, Brown CK, Holtzman MP, et al. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:385–94.
Isambert N, Correia M, Cercueil JP, et al. Hepatic arterial infusion of cisplatin diluted in hypotonic 25 g/1 glucose solution administered in balloon-occluded hepatic artery: experimental rationale and clinical pilot study. J Exp Clin Cancer Res. 2001;20:183–8.
Smith E, Brock AP. The effect of reduced osmolarity on platinum drug toxicity. Br J Cancer. 1989;59:873–5.
Tsujitani S, Fukuda K, Saito H, et al. The administration of hypotonic intraperitoneal cisplatin during operation as a treatment for the peritoneal dissemination of gastric cancer. Surgery. 2002;131:S98–104.
Katano K, Tsujitani S, Oka S, et al. Pharmacokinetics of hypotonic cisplatin chemotherapy administered into the peritoneal and the pleural cavities in experimental model. Anticancer Res. 2000;20:1603–7.
Tsujitani S, Oka A, Kondo A, et al. Administration in a hypotonic solution is preferable to dose escalation in intraperitoneal cisplatin chemotherapy for peritoneal carcinomatosis in rats. Oncology. 1999;57:77–82.
Kondo A, Maeta M, Oka A, et al. Hypotonic intraperitoneal cisplatin chemotherapy for peritoneal carcinomatosis in mice. Br J Cancer. 1996;73:1166–70.
van Etten B, de Vries MR, van IJken MG, et al. Degree of tumour vascularity correlates with drug accumulation and tumour response upon TNF-alpha-based isolated hepatic perfusion. Br J Cancer. 2003;88:314–9.
Savier E, Azoulay D, Huguet E, et al. Percutaneous isolated hepatic perfusion for chemotherapy. A phase I study. Arch Surg. 2003;138:325–32.
Verhoef C, de Wilt JH, Brunstein F, et al. Isolated hypoxic hepatic perfusion with retrograde outflow in patients with irresectable liver metastases: a new simplified technique in isolated hepatic perfusion. Ann Surg Oncol. 2008;15:1367–74.
Jungraithmayr W, Szarzynski M, Neeff H, et al. Significance of total vascular exclusion for hepatic cryotherapy: an experimental study. J Surg Res. 2004;116:32–41.
Miao N, Pingpank JF, Alexander HR, et al. Percutaneous hepatic perfusion in patients with metastatic liver cancer: anesthetic, hemodynamic, and metabolic considerations. Ann Surg Oncol. 2008;15:815–23.
Sato T, Asanuma Y, Kusano T, et al. Difference in hepatic tissue oxygenation between total vascular exclusion and inflow occlusion of the liver and the possible role of hepatic venous blood under liver ischemia. Dig Surg. 1998;15:15–20.
Hiratsuka K, Kim YI, Nakashima K, et al. Tissue oxygen pressure during prolonged ischemia of the liver. J Surg Res. 2000;92:250–4.
Dinant S, Roseboom HJ, Levi M, van Vliet AK, van Gulik TM. Hypothermic in situ perfusion of the porcine liver using Celsior or Ringer-lactate solution. Langenbecks Arch Surg. 2009;394:143–50.
Heijnen BHM, Straatsburg ICH, Gouma DJ, van Gulik TM. Decrease in core liver temperature with 10°C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs. Surgery. 2003;134:806–17.
Petrowsky H, McCormack L, Trujillo M, et al. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–30.
Saidi RF, Chang J, Verb S, et al. The effect of methylprednisolone on warm ischemia-reperfusion injury in the liver. Am J Surg. 2007;193:345–8.
Acknowledgment
Supported in part by grants from the French National League against Cancer (Committees of Côte d’Or, Saône et Loire and Nièvre) and from the Scientific Committee of the School of Medicine of Dijon (University of Burgundy, France). We thank Jean-Luc Beltramo for measuring platinum concentrations, Laurence Duvillard for determining osmolarity, Pierre-Emmanuel Puig for the in vitro assays, Philippe Pointaire and Jean-Mathieu Ricard for their help during the experiments in the pigs, and Philip Bastable for revising the article in manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortega-Deballon, P., Facy, O., Consolo, D. et al. Hypoxic Single-Pass Isolated Hepatic Perfusion of Hypotonic Cisplatin: Safety Study in the Pig. Ann Surg Oncol 17, 898–906 (2010). https://doi.org/10.1245/s10434-009-0775-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0775-z