ABSTRACT
Introduction
The clinical significance of isolated tumor cells (ITC) in peripheral blood (PB) and bone marrow (BM) as predictive markers in the recurrence or metastasis of breast cancer has not yet been determined. In the current study, we focused on the urokinase plasminogen activator receptor (u-PAR) gene as a powerful indicator of the potential to relapse after surgery.
Patients and Methods
We examined CK-7 and CK19 as an ITC marker and u-PAR as a candidate indicator for metastasis in PB and BM from 800 cases of breast cancer by quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR). Serum tumor markers, carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3), were compared with u-PAR or CK status.
Results
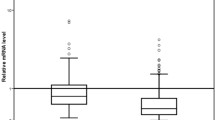
CK7 in PB was positive in 262 cases that showed a poorer disease-free survival (DFS) than 478 CK7(–) cases (P < 0.05). The 153 cases of u-PAR(+) in BM showed significantly poorer DFS and overall survival (OS) than did the 579 cases of u-PAR(–) in BM (P < 0001 and P < 0.0001, respectively). In PB, a significant difference was also observed between 330 cases of u-PAR(+) and 437 cases of u-PAR(–) (P < 0.0001). The hazard ratio (HR) for prediction of recurrence was significantly higher in u-PAR (P < 0.0001; HR 0.0519) than the level of three serum tumor markers.
Discussion
u-PAR expresses in cancer cells during the dormant phase. The current findings revealed that the expression levels of u-PAR in PB and BM evaluated preoperatively indicate the potential to relapse or metastasize after surgery.




Similar content being viewed by others
References
Braun S, Cevatli BS, Assemi C, Janni W, Kentenich CR, Schindlbeck C, et al. Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol. 2001;19:1468–75.
Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. New Eng J Med. 2000;342:525–33.
Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC. Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet. 1999;354:197–202.
Masuda N, Tamaki Y, Sakita I, et al. Clinical significance of micrometastases in axillary lymph nodes assessed by reverse transcription-polymerase chain reaction in breast cancer patients. Clin Cancer Res. 2000;6:4176–85.
Molino A, Micciolo R, Turazza M, et al. Prognostic significance of estrogen receptors in 405 primary breast cancers: a comparison of immunohistochemical and biochemical methods. Breast Cancer Res Treat. 1997;45:241–9.
Salvadori B, Squicciarini P, Rovini D, et al. Use of monoclonal antibody MBr1 to detect micrometastases in bone marrow specimens of breast cancer patients. Eur J Cancer. 1990;26:865–7.
Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. New Eng J Med. 2005;353:793–802.
Masuda TA, Kataoka A, Ohno S, et al. Detection of occult cancer cells in peripheral blood and bone marrow by quantitative RT-PCR assay for cytokeratin-7 in breast cancer patients. Int J Oncol. 2005;26:721–30.
Heiss MM, Allgayer H, Gruetzner KU, et al. Individual development and uPA-receptor expression of disseminated tumour cells in bone marrow: a reference to early systemic disease in solid cancer. Nat Med. 1995;1:1035–9.
Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98.
Brackstone M, Townson JL, Chambers AF. Tumour dormancy in breast cancer: an update. Breast Cancer Res. 2007;9:208.
Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol. 2007;4:699–710.
Laufs S, Schumacher J, Allgayer H. Urokinase-receptor (u-PAR): an essential player in multiple games of cancer: a review on its role in tumor progression, invasion, metastasis, proliferation/dormancy, clinical outcome and minimal residual disease. Cell Cycle. Georgetown, Tex 2006;5:1760–71.
Iinuma H, Okinaga K, Egami H, et al. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306.
Holmgren L, O’Reilly M, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53.
Murray C. Tumour dormancy: not so sleepy after all. Nat Med. 1995;1:117–8.
Pantel K, Brakenhoff R Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56.
Uhr J, Scheuermann R, Street N, Vitetta E. Cancer dormancy: opportunities for new therapeutic approaches. Nat Med. 1997;3:505–9.
Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Oncol. 2008;123:1991–2006.
Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. Apmis. 2008;116:754–70.
Allgayer H, Aguirre-Ghiso JA. The urokinase receptor (u-PAR)–a link between tumor cell dormancy and minimal residual disease in bone marrow? Apmis. 2008;116:602–14.
Mimori K, Fukagawa T, Kosaka Y, et al. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609–16.
Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–2.
Hildenbrand R, Glienke W, Magdolen V, Graeff H, Stutte HJ, Schmitt M. Urokinase receptor localization in breast cancer and benign lesions assessed by in situ hybridization and immunohistochemistry. Histochem Cell Biol. 1998;110:27–32.
Schlimok G, Funke I, Pantel K, et al. Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer. 1991;27:1461–5.
Dubuisson L, Monvoisin A, Nielsen BS, Le Bail B, Bioulac-Sage P, Rosenbaum J. Expression and cellular localization of the urokinase-type plasminogen activator and its receptor in human hepatocellular carcinoma. J Pathol. 2000;190:190–5.
Pyke C, Graem N, Ralfkiaer E, et al. Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res. 1993;53:1911–5.
Acknowledgement
This work was supported by the following grant sponsors: CREST, Japan Science and Technology Agency (JST); Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research, grant numbers 17109013, 17591411, 17591413, 18390367, 18590333, 18659384, and 18790964; The Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Grant-in-Aid for Scientific Research on Priority Areas, grant number 18015039; and the Third Term Comprehensive Ten-Year Strategy for Cancer Control, grant number 16271201.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mimori, K., Kataoka, A., Yamaguchi, H. et al. Preoperative u-PAR Gene Expression in Bone Marrow Indicates the Potential Power of Recurrence in Breast Cancer Cases. Ann Surg Oncol 16, 2035–2041 (2009). https://doi.org/10.1245/s10434-009-0465-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0465-x