Abstract
Objectives
Atypical protein kinase C iota (aPKC-ι) and its associated intracellular molecules, E-cadherin and β-catenin, are important for cell polarization in tumorigenesis and progression. Expression of aPKC-ι, P-aPKC-ι (activated aPKC-ι), E-cadherin, and β-catenin in hepatocellular carcinoma (HCC) was measured, and correlation with clinicopathological characteristics of HCC was analyzed.
Methods
Paraffin-embedded tumor tissue was obtained from patients with HCC after resection without preoperative radiotherapy or chemotherapy. Gene expression was detected by polymerase chain reaction (PCR), and protein expression was detected by immunohistochemistry and Western blot analysis. Expressions of aPKC-ι, P-aPKC-ι, E-cadherin, and β-catenin were analyzed with relation to the clinicopathological data.
Results
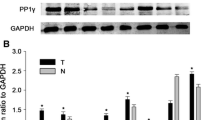
The gene and protein expression of aPKC-ι are obviously higher in HCC tissues than that in peritumoral tissues and normal tissues by semiquantitative PCR and immunohistochemistry methods. Accumulation of aPKC-ι in HCC cytoplasm and nucleolus inhibited the later formation of belt-like adherens junctions (AJs) and/or tight junctions (TJs) in cell–cell contact. E-cadherin was reduced and accumulation of cytoplasm β-catenin was increased in HCC. The expression of aPKC-ι was closely related to pathological differentiation, tumor size, invasion, and metastasis of HCC.
Conclusion
Accumulation of cytoplasm aPKC-ι may reflect pathological differentiation, invasion, and metastasis potential of HCC. In this regard, our study on HCC revealed the potential usefulness of aPKC-ι, E-cadherin, and β-catenin as a prognostic marker, closely related to pathological differentiation, invasion, metastasis, and prognosis of HCC.


Similar content being viewed by others
References
Suzuki A, Akimoto K, Ohno S. Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J Biochem. 2003;133(1):9–16.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58.
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100.
Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275(31):24136–45.
Roberson ED, English JD, Sweatt JD. A biochemist’s view of long-term potentiation. Learning & memory. Cold Spring Harbor: NY; 1996. 3(1), p 1-24.
Ashida N, Ueyama T, Rikitake K, et al. Ca(2+) oscillation induced by P2Y2 receptor activation and its regulation by a neuron-specific subtype of PKC (gammaPKC). Neurosci Lett. 2008;446(2–3):123–8.
Eder AM, Sui X, Rosen DG, et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(35):12519–24.
Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase C iota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280(35):31109–15.
Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development (Cambridge, England). 1998;125(18):3607–14.
Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339(8807):1453–7.
Ikeguchi M, Makino M, Kaibara N. Clinical significance of E-cadherin-catenin complex expression in metastatic foci of colorectal carcinoma. J Surg Oncol. 2001;77(3):201–7.
Ranscht B. Cadherins and catenins: interactions and functions in embryonic development. Curr Opin Cell Biol. 1994;6(5):740–6.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54.
Sillem M, Hahn U, 3rd CC, Gordon K, Runnebaum B, Hodgen G. Ectopic growth of endometrium depends on its structural integrity and proteolytic activity in the cynomolgus monkey (Macaca fascicularis) model of endometriosis. Fertil Steril. 1998;66(3):468–73.
Hira E, Ono T, Dhar DK, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer. 2005;103(3):588–98.
Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274(1 Pt 2):F1–9.
Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79(1):73–98.
Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1999;179(2):115–25.
Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108(Pt 1):127–42.
Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell. 2000;100(2):209–19.
Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87(8):992–1005.
Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77(8 Suppl):1605–13.
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–3.
Wijnhoven BP, Pignatelli M, Dinjens WN, Tilanus HW. Reduced p120ctn expression correlates with poor survival in patients with adenocarcinoma of the gastroesophageal junction. J Surg Oncol. 2005;92(2):116–23.
Saha B, Kaur P, Tsao-Wei D, et al. Unmethylated E-cadherin gene expression is significantly associated with metastatic human prostate cancer cells in bone. Prostate. 2008;68(15):1681–8.
Chetty R, Serra S, Asa SL. Loss of membrane localization and aberrant nuclear E-cadherin expression correlates with invasion in pancreatic endocrine tumors. Am J Surg Pathol. 2008;32(3):413–9.
Esufali S, Charames GS, Pethe VV, Buongiorno P, Bapat B. Activation of tumor-specific splice variant Rac1b by dishevelled promotes canonical Wnt signaling and decreased adhesion of colorectal cancer cells. Cancer Res. 2007;67(6):2469–79.
Totong R, Achilleos A, Nance J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development (Cambridge, England). 2007;134(7):1259–68.
Suzuki A, Hirata M, Kamimura K, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14(16):1425–35.
Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci. 2002;115(Pt 18):3565–73.
Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13(5):641–8.
Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol. 2001;11(7):482–8.
Kay AJ, Hunter CP. CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Curr Biol 2001;11(7):474–81.
Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106(4):489–98.
Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421(6924):753–6.
Li L, Yuan H, Weaver CD, et al. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18(15):4233–40.
Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296(5573):1644–6.
Acknowledgement
This work was supported by grant no. 30672040 to Professor Jianming Wang, M.D. from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, GS., Wang, JM., Lu, JX. et al. Expression of P-aPKC-ι, E-Cadherin, and β-Catenin Related to Invasion and Metastasis in Hepatocellular Carcinoma. Ann Surg Oncol 16, 1578–1586 (2009). https://doi.org/10.1245/s10434-009-0423-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0423-7