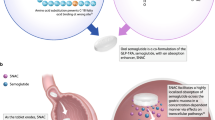
Abstract
Rosuvastatin is an efficient antihyperlipidemic agent; however, being a BCS class II molecule, it shows poor oral bioavailability of < 20%. The present study focused on the improvement of oral bioavailability of rosuvastatin using tailored niosomes. The niosomes were prepared by film hydration method and sonication using cholesterol and Span 40. The Box-Behnken design (BBD) was applied to optimize the size (98 nm) and the entrapment efficacy (77%) of the niosomes by selecting cholesterol at 122 mg, Span 40 at 0.52%, and hydration time at 29.88 min. The transmission electron microscopy image showed spherical shape niosomes with smooth surface without aggregation. The ex vivo intestinal permeability studies showed significant improvement in the rosuvastatin permeation (95.5% after 2 h) using niosomes in comparison to the rosuvastatin suspension (40.1% after 2 h). The in vivo pharmacokinetic parameters in the rat model confirmed the improvement in the oral bioavailability with optimized rosuvastatin loaded niosomes (relative bioavailability = 2.01) in comparison to the rosuvastatin suspension, due to high surface area of niosomes and its lymphatic uptake via transcellular route. In conclusion, the optimized rosuvastatin loaded niosomes offers a promising approach to improve the oral bioavailability of rosuvastatin.



Similar content being viewed by others
Data Availability
The raw data are available on request to the corresponding author.
Change history
11 July 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1208/s12249-022-02348-z
References
Awad HH, Anderson FA Jr, Gore JM, Goodman SG, Goldberg RJ. Cardiogenic shock complicating acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. Am Heart J. 2012;163(6):963–71.
J.A. Ambrose, M. Singh, Pathophysiology of coronary artery disease leading to acute coronary syndromes, Fprime reports 7 (2015).
Turpie AG. Burden of disease: medical and economic impact of acute coronary syndromes. Am J Manag Care. 2006;12(16):S430.
Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. Jama. 2002;288(4):462–7.
Heeschen C, Hamm CW, Laufs U, Snapinn S, Böhm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–52.
Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25(10):2553–63.
Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822–35.
Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10(1):11–28.
Agouridis A, Tsimihodimos V, Filippatos T, Tselepis A, Elisaf M. High doses of rosuvastatin are superior to low doses of rosuvastatin plus fenofibrate or n-3 fatty acids in mixed dyslipidemia. Lipids. 2011;46(6):521–8.
Shilakari Asthana G, Sharma PK, Asthana A. In vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Scientifica. 2016;2016:1–10.
Zarkesh K, Khazaeli P, Pardakhty A, Rezaifar M. Preparation and physicochemical characterization of topical niosomal formulation of minoxidil and tretinoin. Global J Pharm Pharm Sci. 2017;3(2):21–6.
Kazi KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M, et al. Niosome: a future of targeted drug delivery systems. J Adv Pharm Tech Res. 2010;1(4):374.
Wang G, Wang J, Wu W, S.S. Tony To, Zhao H, Wang J. Advances in lipid-based drug delivery: enhancing efficiency for hydrophobic drugs. Expert Opin Drug Deliv. 2015;12(9):1475–99.
Khan R, Irchhaiya R. Niosomes: a potential tool for novel drug delivery. J Pharm Investig. 2016;46(3):195–204.
Madhav S, Gupta D. A review on microemulsion based system. Int J Pharm Sci Res. 2011;2(8):1888.
Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12(11):1561–72.
Muzaffar F, Singh U, Chauhan L. Review on microemulsion as futuristic drug delivery. Int J Pharm Pharm Sci. 2013;5(3):39–53.
Gabr MM, Mortada SM, Sallam MA. Carboxylate cross-linked cyclodextrin: a nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur J Pharm Sci. 2018;111:1–12.
Balakumar K, Raghavan CV, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B: Biointerfaces. 2013;112:337–43.
Kamel AO, Mahmoud AA. Enhancement of human oral bioavailability and in vitro antitumor activity of rosuvastatin via spray dried self-nanoemulsifying drug delivery system. J Biomed Nanotechnol. 2013;9(1):26–39.
Abo Enin HA. Self-nanoemulsifying drug-delivery system for improved oral bioavailability of rosuvastatin using natural oil antihyperlipdemic. Drug Dev Ind Pharm. 2015;41(7):1047–56.
Gadad AP, Tigadi SG, Dandagi PM, Mastiholimath VS, Bolmal UB. Rosuvastatin loaded nanostructured lipid carrier: For enhancement of oral bioavailability. Indian J Pharm Educ Res. 2016;50(4):605–11.
Alshora DH, Ibrahim MA, Elzayat E, Almeanazel OT, Alanazi F. Rosuvastatin calcium nanoparticles: improving bioavailability by formulation and stabilization codesign. PLoS One. 2018;13(7):e0200218.
Gabr MM, Mortada SM, Sallam MA. Hexagonal liquid crystalline nanodispersions proven superiority for enhanced oral delivery of rosuvastatin: in vitro characterization and in vivo pharmacokinetic study. J Pharm Sci. 2017;106(10):3103–12.
Hirpara MR, Manikkath J, Sivakumar K, Managuli RS, Gourishetti K, Krishnadas N, et al. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: development and in vitro and in vivo evaluations. Int J Biol Macromol. 2018;107:2190–200.
Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6(8):813–25.
Lohani A, Verma A. Vesicles: potential nano carriers for the delivery of skin cosmetics. J Cosmet Laser Ther. 2017;19(8):485–93.
Castro GA, Ferreira LA. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin Drug Deliv. 2008;5(6):665–79.
Hamishehkar H, Rahimpour Y, Kouhsoltani M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin Drug Deliv. 2013;10(2):261–72.
Mahale N, Thakkar P, Mali R, Walunj D, Chaudhari S. Niosomes: novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interf Sci. 2012;183:46–54.
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185:22–36.
Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15.
Schroeter A, Engelbrecht T, Neubert RH, Goebel AS. New nanosized technologies for dermal and transdermal drug delivery. A review. J Biomed Nanotechnol. 2010;6(5):511–28.
Ibrahim MM, Nair AB, Aldhubiab BE, Shehata TM. Hydrogels and their combination with liposomes, niosomes, or transfersomes for dermal and transdermal drug delivery. Liposomes. 2017;155.
Salih OS, Samein LH, Ali WK. Formulation and in vitro evaluation of rosuvastatin calcium niosomes. Int J Pharm Pharm Sci. 2013;5(4):525–35.
Rezaeiroshan A, Saeedi M, Morteza-Semnani K, Akbari J, Gahsemi M, Nokhodchi A. Development of trans-Ferulic acid niosome: an optimization and an in-vivo study. J Drug Deliv Sci Technol. 2020;59:101854.
Tabbakhian M, Tavakoli N, Jaafari MR, Daneshamouz S. Enhancement of follicular delivery of finasteride by liposomes and niosomes: 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. Int J Pharm. 2006;323(1-2):1–10.
Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377(1-2):1–8.
Manca ML, Manconi M, Nacher A, Carbone C, Valenti D, Maccioni AM, et al. Development of novel diolein–niosomes for cutaneous delivery of tretinoin: Influence of formulation and in vitro assessment. Int J Pharm. 2014;477(1-2):176–86.
Yeo LK, Chaw CS, Elkordy AA. The effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals. 2019;12(2):46–56.
Maulvi FA, Parmar RJ, Desai AR, Desai DM, Shukla MR, Ranch KM, et al. Tailored gatifloxacin Pluronic® F-68-loaded contact lens: addressing the issue of transmittance and swelling. Int J Pharm. 2020;119279.
Ferreira SC, Bruns R, Ferreira H, Matos G, David J, Brandao G, et al. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–86.
S. Bhaskaran, P. Lakshmi, Comparative evaluation of niosome formulations prepared by different techniques, Acta Pharm, aceutica Sciencia 32 (2009).
Girigoswami A, Das S, De S. Fluorescence and dynamic light scattering studies of niosomes-membrane mimetic systems. Spectrochimica Acta Part A: Molecular Biomolecular Spectroscopy. 2006;64(4):859–66.
Kumar TR, Shitut NR, Kumar PK, Vinu MC, Kumar VVP, Mullangi R, et al. Determination of rosuvastatin in rat plasma by HPLC: validation and its application to pharmacokinetic studies. Biomed Chromatogr. 2006;20(9):881–7.
Kaila H, Ambasana M, Thakkar R, Saravaia H, Shah A. A new improved RP-HPLC method for assay of rosuvastatin calcium in tablets. Indian J Pharm Sci. 2010;72(5):592–8.
Pandey SS, Maulvi FA, Patel PS, Shukla MR, Shah KM, Gupta AR, et al. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: in vitro and in vivo studies. Colloids Surf B: Biointerfaces. 2020;186:110681.
Maulvi FA, Pillai LV, Patel KP, Desai AR, Shukla MR, Desai DT, et al. Lidocaine tripotassium phosphate complex laden microemulsion for prolonged local anaesthesia: in vitro and in vivo studies. Colloids Surf B: Biointerfaces. 2020;185:110632.
Pandey SS, Patel MA, Desai DT, Patel HP, Gupta AR, Joshi SV, et al. Bioavailability enhancement of repaglinide from transdermally applied nanostructured lipid carrier gel: optimization, in vitro and in vivo studies. J Drug Deliv Sci Technol. 2020;57:101731.
Pandey S, Swamy SV, Gupta A, Koli A, Patel S, Maulvi F, et al. Multiple response optimisation of processing and formulation parameters of pH sensitive sustained release pellets of capecitabine for targeting colon. J Microencapsul. 2018;35(3):259–71.
Desai AR, Maulvi FA, Desai DM, Shukla MR, Ranch KM, Vyas BA, et al. Multiple drug delivery from the drug-implants-laden silicone contact lens: addressing the issue of burst drug release. Mater Sci Eng C. 2020;110885.
Lin JH, Sugiyama Y, Awazu S, Hanano M. In vitro andin vivo evaluation of the tissue-to-blood partition coefficient for physiological pharmacokinetic models. J Pharmacokinet Biopharm. 1982;10(6):637–47.
Mirfazaelian A, Kim K-B, Anand SS, Kim HJ, Tornero-Velez R, Bruckner JV, et al. Development of a physiologically based pharmacokinetic model for deltamethrin in the adult male Sprague-Dawley rat. Toxicol Sci. 2006;93(2):432–42.
Maulvi FA, Bodaa AM, Desai AR, Choksi HH, Ranch KM, Shah DO. Application of Box-Behnken design in optimization of ibuprofen ternary solid dispersion. J Pharm Appl Sci. 2015;2(2):1–11.
Nasseri B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int J Pharm. 2005;300(1-2):95–101.
Barani M, Nematollahi MH, Zaboli M, Mirzaei M, Torkzadeh-Mahani M, Pardakhty A, et al. In silico and in vitro study of magnetic niosomes for gene delivery: the effect of ergosterol and cholesterol. Mater Sci Eng C. 2019;94:234–46.
Arunachalam A, Jeganath S, Yamini K, Tharangini K. Niosomes: a novel drug delivery system. Int J Novel Trends Pharm Sci. 2012;2(1):25–31.
Devaraj GN, Parakh S, Devraj R, Apte S, Rao BR, Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J Colloid Interface Sci. 2002;251(2):360–5.
Damera DP, Venuganti VVK, Nag A. Deciphering the role of bilayer of a niosome towards controlling the entrapment and release of dyes. Chemistry. 2018;3(14):3930–8.
Sun J, Wang F, Sui Y, She Z, Zhai W, Wang C, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine. 2012;7:5733.
Junyaprasert VB, Teeranachaideekul V, Supaperm T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech. 2008;9(3):851–61.
Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Ciprofloxacin nano-niosomes for targeting intracellular infections: an in vitro evaluation. J Nanopart Res. 2013;15(4):1556.
Vishwakarma N, Jain A, Sharma R, Mody N, Vyas S, Vyas SP. Lipid-based nanocarriers for lymphatic transportation. AAPS PharmSciTech. 2019;20(2):83.
Siegel I, Gordon H. Surfactant-induced increases of permeability of rat oral mucosa to non-electrolytes in vivo. Arch Oral Biol. 1985;30(1):43–7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors have no potential conflict of interest to disclose this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1208/s12249-022-02348-z
Supplementary Information
ESM 1
(DOCX 356 kb)
About this article
Cite this article
Liu, Q., Xu, J., Liao, K. et al. RETRACTED ARTICLE: Oral Bioavailability Improvement of Tailored Rosuvastatin Loaded Niosomal Nanocarriers to Manage Ischemic Heart Disease: Optimization, Ex Vivo and In Vivo Studies. AAPS PharmSciTech 22, 58 (2021). https://doi.org/10.1208/s12249-021-01934-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01934-x