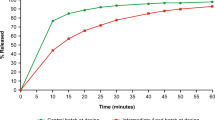
Abstract
To estimate strength of a scopolamine transdermal delivery system (TDS) in vivo, using residual drug vs. pharmacokinetic analyses with the goal of scientifically supporting a single and robust method for use across the dosage form and ultimately facilitate the development of more consistent and clinically meaningful labeling. A two-arm, open-label, crossover pharmacokinetic study was completed in 26 volunteers. Serum samples were collected and residual scopolamine was extracted from worn TDS. Delivery extent and rate were estimated by (1) numeric deconvolution and (2) steady-state serum concentration determined from graphical and non-compartmental analyses. In residual drug analyses, mean ± SD scopolamine release rate was 0.015 ± 0.002 mg/h (11% RSD), vs. 0.016 ± 0.006 mg/h (35% RSD) from numeric deconvolution, 0.015 ± 0.005 mg/h (34% RSD) from graphical analysis, and 0.015 ± 0.007 mg/h (44% RSD) from non-compartmental analysis. In residual drug analyses, total drug released was 1.09 ± 0.11 mg (10% RSD), vs. 1.12 ± 0.40 mg (35% RSD) from numeric deconvolution, 1.07 ± 0.35 mg (33% RSD) from graphical analysis, and 1.07 ± 0.45 (42% RSD) from non-compartmental analysis. Extent and rate of scopolamine release were comparable by both approaches, but pharmacokinetic analysis demonstrated greater inter-subject variability.


Similar content being viewed by others
Abbreviations
- TDS:
-
Transdermal delivery system
References
Strasinger C, Raney SG, Tran DC, Ghosh P, Newman B, Bashaw ED, et al. Navigating sticky areas in transdermal product development. J Control Release. 2016;233:1–9.
Transderm Scōp® package insert. In: Alza C, editor. Vacaville, CA.2017.
Farage MA, Maibach HI, Andersen KE, Lachapelle JM, Kern P, Ryan C, et al. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermatitis. 2011;65(2):65–75.
FDA. Transdermal and Topical Delivery Systems – Product development and quality considerations draft guidance for industry. 2019. Available from: https://www.fda.gov/media/132674/download Last accessed March 6, 2020.
John H, Binder T, Hochstetter H, Thiermann H. LC-ESI MS/MS quantification of atropine and six other antimuscarinic tropane alkaloids in plasma. Anal Bioanal Chem. 2010;396(2):751–63.
Swaminathan SK, Fisher J, Brogden NK, Kandimalla KK. Development and validation of a sensitive LC-MS/MS method for the estimation of scopolamine in human serum. J Pharm Biomed Anal. 2018;164:41–6.
Schwarz G. Quantitative analysis of activation and inactivation of asymmetry currents in biological membranes, based on a conformational transition model. J Membr Biol. 1978;43(2–3):149–67.
Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2(2):123–48.
Bourne, DWA. Mathematical modeling of pharmacokinetic data. (Technomic Publishing Co., Inc: Lancaster, PA, 1995).
Csizmadia F, Endrenyi L. Model-independent estimation of lag times with first-order absorption and disposition. J Pharm Sci. 1998;87(5):608–12.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd edn. CRC Press (Taylor and Francis Group, Boca Raton, FL, 1982).
Kim YC, Song JM, Lipatov AS, Choi SO, Lee JW, Donis RO, et al. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur J Pharm Biopharm eV. 2012;81(2):239–47.
Pubchem. [Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5184.] Last accessed April 8, 2018.
Chandrashekar NS, Shobha Rani RH. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J Pharm Sci. 2008;70(1):94–6.
Putcha L, Cintron NM, Tsui J, Vanderploeg JM, Kramer WG. Pharmacokinetics and oral bioavailability of scopolamine in normal subjects. Pharm Res. 1989;6(6):481–5.
Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41(1):51–60.
Nachum Z, Shupak A, Gordon CR. Transdermal scopolamine for prevention of motion sickness : clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet. 2006;45(6):543–66.
Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998;38(8):720–6.
Kanto J, Kentala E, Kaila T, Pihlajamaki K. Pharmacokinetics of scopolamine during caesarean section: relationship between serum concentration and effect. Acta Anaesthesiol Scand. 1989;33(6):482–6.
Singh I, Morris AP. Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig. 2011;1(1):4–9.
Funding
This project was funded by the following grants: National Institute for Pharmaceutical Technology and Education U01 Critical Patch Manufacturing Sector Research Initiative (5U01FD004275), and NIH/NCATS CTSA U54TR001356.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Disclaimer
Views expressed in this manuscript do not necessarily reflect official policies of the United States Food and Drug Administration; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Additional information
Guest Editors: Ajaz S. Hussain, Kenneth Morris, and Vadim J. Gurvich
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Swaminathan, S.K., Strasinger, C., Kelchen, M. et al. Determination of Rate and Extent of Scopolamine Release from Transderm Scōp® Transdermal Drug Delivery Systems in Healthy Human Adults. AAPS PharmSciTech 21, 117 (2020). https://doi.org/10.1208/s12249-020-01658-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01658-4