Abstract
The objective of the current study was to develop ziprasidone hydrochloride monohydrate (ZHM) nanocrystal–based orally dispersible tablet (ODT) formulations. Design of experiment approach was used to develop ODTs. The tablets were compressed using direct compression method and characterized with quality control tests. In vitro dissolution studies and Caco-2 cell permeability tests were executed. The hardness and friability values of nanocrystal-based ODTs were found 31.2 N and 1.05%, respectively. The disintegration time was below 10 s. Dissolution profile in pH 7.4 phosphate buffer showed that nanocrystal-based ODTs and commercial product were dissolved in 120 min 58.98% and 16%, respectively. In pH 7.4 phosphate buffer with SLS, sample groups dissolved above 85% at the end of the study. Permeability value and cumulative ZHM amount on the cells were improved with nanocrystals. In conclusion, the novel formulation of ZHM nanocrystal–based ODTs was successfully developed for alternative dosage form.










Similar content being viewed by others
Change history
19 May 2020
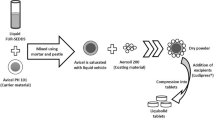
Several typos occurred during the production process and captions were misplaced. The corrected captions for Picture 1, Fig. 6-9 are below.
References
Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Schizophr Res 2016
Bobes J, Cañas F, Rejas J, MacKell J. Economic consequences of the adverse reactions related with antipsychotics: an economic model comparing tolerability of ziprasidone, olanzapine, risperidone, and haloperidol in Spain. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:1287–97.
Jablensky A. The 100-year epidemiology of schizophrenia. Schizophr Res. 1997.
Jones P, Cannon M. The new epidemiology of schizophrenia. Psychiatr Clin North Am. 1998
Mullins CD, Shaya FT, Zito JM, Obeidat N, Naradzay J, Harrison DJ. Effect of initial ziprasidone dose on treatment persistence in schizophrenia. Schizophr Res. 2006
Kozlowski-Gibson M. The struggle for schizophrenia treatment: a case study. Int J Law Psychiatry 2016
Harvey PD, Rosenthal JB. Treatment resistant schizophrenia: course of brain structure and function. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2016.
San L, Casillas M, Ciudad A, Gilaberte I. Olanzapine orally disintegrating tablet: a review of efficacy and compliance. CNS Neurosci Ther. 2008;14:203–14.
Kinon BJ, Hill AL, Liu H, Kollack-Walker S. Erratum: Olanzapine orally disintegrating tablets in the treatment of acutely ill non-compliant patients with schizophrenia. Int J Neuropsychopharmacol (International Journal of Neuropsychopharmacology). 2003;6(2):97–102 6:313.
Hackl E, Ermolina I. Application of texture analysis technique in formulation development of lyophilized orally disintegrating tablets containing mannitol, polyvinylpyrrolidone and amino acids. AAPS PharmSciTech. 2019;20:1–16.
Siddiqui N, Garg G, Sharma PK. Fast dissolving tablets: preparation, characterization and evaluation: an overview. Int J Pharm Sci Rev Res 2010
Slavkova M, Breitkreutz J. Orodispersible drug formulations for children and elderly. Eur J Pharm Sci. 2015.
Song Q, Guo X, Sun Y, Yang M. Anti-solvent precipitation method coupled electrospinning process to produce poorly water-soluble drug-loaded orodispersible films. AAPS PharmSciTech. 2019;20:1–11.
Novick D, Montgomery W, Treuer T, Koyanagi A, Aguado J, Kraemer S, et al. Comparison of clinical outcomes with orodispersible versus standard oral olanzapine tablets in nonadherent patients with schizophrenia or bipolar disorder. Patient Prefer Adherence. 2017;11:1019–25.
Ascher-Svanum H, Furiak NM, Lawson AH, Klein TM, Smolen LJ, Conley RR, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United States. J Med Econ. 2012;15:531–47.
Zakowiecki D, Cal K, Kaminski K, Adrjanowicz K, Swinder L, Kaminska E, et al. The improvement of the dissolution rate of ziprasidone free base from solid oral formulations. AAPS PharmSciTech. 2015;16:922–33.
Mattei C, Rapagnani MP, Stahl SM. Ziprasidone hydrocloride: what role in the management of schizophrenia? J Cent Nerv Syst Dis. 2011;3:1–16.
Patil JS, Korachagaon AV, Shiralashetti SS, Marapur SC. Enhancing dissolution rate of ziprasidone via co-grinding technique with highly hydrophilic carriers. RGUHS J Pharm Sci. 2012;2:26.
Thombre AG, Shah JC, Sagawa K, Caldwell WB. In vitro and in vivo characterization of amorphous, nanocrystalline, and crystalline ziprasidone formulations. Int J Pharm [Internet]. Elsevier B.V.; 2012;428:8–17. Available from: https://doi.org/10.1016/j.ijpharm.2012.02.004.
Dening TJ, Rao S, Thomas N, Prestidge CA. Silica encapsulated lipid-based drug delivery systems for reducing the fed/fasted variations of ziprasidone in vitro. Eur J Pharm Biopharm. 2016;101:33–42.
Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43–8.
Tashan E, Karakucuk A, Celebi N. Optimization and in vitro evaluation of ziprasidone nanosuspensions produced by a top-down approach. J Drug Deliv Sci Technol [Internet]. Elsevier; 2019;52:37–45. Available from: https://doi.org/10.1016/j.jddst.2019.04.024.
Karakucuk A, Celebi N, Teksin ZS. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A design of experiment approach. Eur J Pharm Sci [Internet]. Elsevier B.V.; 2016;95:111–21. Available from: https://doi.org/10.1016/j.ejps.2016.05.010.
Oktay AN, Karakucuk A, Ilbasmis-Tamer S, Celebi N. Dermal flurbiprofen nanosuspensions: optimization with design of experiment approach and in vitro evaluation. Eur J Pharm Sci. 2018;122:254–63.
Taşhan E, Karaküçük A, Çelebi N. Orally disintegrating tablets: general overview and Quality by Design approach: review. Turkiye Klin J Pharm Sci. 2017;6:43–58.
Gajera BY, Shah DA, Dave RH. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. Int J Pharm [Internet]. Elsevier; 2019;559:348–59. Available from: https://doi.org/10.1016/j.ijpharm.2019.01.054.
Ibrahim AH, Rosqvist E, Smått JH, Ibrahim HM, Ismael HR, Afouna MI, et al. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int J Pharm [Internet]. Elsevier; 2019;563:217–27. Available from: https://doi.org/10.1016/j.ijpharm.2019.03.064.
Usman S, Ejaz RR, Safdar KA. Formulation development and optimization of orally disintegrating tablets of montelukast sodium by design-expert. Trop J Pharm Res. 2018;17:1701–9.
Basalious EB, El-Sebaie W, El-Gazayerly O. Rapidly absorbed orodispersible tablet containing molecularly dispersed felodipine for management of hypertensive crisis: development, optimization and in vitro/in vivo studies. Pharm Dev Technol. 2013;18:407–16.
Ibrahim AH, Smått J-H, Govardhanam NP, Ibrahim HM, Ismael HR, Afouna MI, et al. Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin. Eur J Pharm Sci [Internet]. Elsevier; 2020;142:105103. Available from: https://doi.org/10.1016/j.ejps.2019.105103.
Sammour OA, Hammad MA, Zidan AS, Mowafy AG. QbD approach of rapid disintegrating tablets incorporating indomethacin solid dispersion. Pharm Dev Technol. 2011
Charoo NA, Shamsher AAA, Zidan AS, Rahman Z. Quality by design approach for formulation development: a case study of dispersible tablets. Int J Pharm [Internet]. Elsevier B.V.; 2012;423:167–78. Available from: https://doi.org/10.1016/j.ijpharm.2011.12.024.
El-Nabarawi MA, El-Miligi MF, Khalil IA, El-Nabarawy NA. Applying QBD approach to develop ODTs containing aceclofenac solid dispersion with ranitidine HCL using direct compression technique, then pharmaceutically evaluating and pharmacologically confirming the therapeutic actions. Int J Pharm Pharm Sci. 2013;5:577–93.
Ali HSM, Hanafy AF, Alqurshi A. Engineering of solidified glyburide nanocrystals for tablet formulation via loading of carriers: downstream processing, characterization, and bioavailability. Int J Nanomedicine. 2019;14:1893–906.
European Pharmacopeia 8th Edition, monograph 2.9.7. Friability of Uncoated Tablets. 2014.
Thulluru A, Palei NN, Vimala M, Vidyasagar M, Vishnupriya K. Quality By Design approach to optimize the taste masked zolpidem tartrate oral disintegrating tablets. Res J Pharm Dos Forms Technol. 2018;10:139.
Nair A, Khunt D, Misra M. Application of quality by design for optimization of spray drying process used in drying of Risperidone nanosuspension. Powder Technol [internet]. Elsevier B.V.; 2019;342:156–65. Available from: https://doi.org/10.1016/j.powtec.2018.09.096.
Prasanthi NL, Manikiran SS, Rao R. Design and evaluation of orodispersible tablets of ziprasidone hydrochloride. Indian Pharm. 2010
Que L, Wu W, Cheng X, Hu T. Evaluation of disintegrating time of rapidly disintegrating tablets by a paddle method. Pharm Dev Technol. 2006
Abouhussein DMN, El Nabarawi MA, Shalaby SH, El-Bary AA. Sertraline-cyclodextrin complex orodispersible sublingual tablet: optimization, stability, and pharmacokinetics. J Pharm Innov. Journal of Pharmaceutical Innovation; 2019
Mishra SM, Rohera BD. An integrated, quality by design (QbD) approach for design, development and optimization of orally disintegrating tablet formulation of carbamazepine. Pharm Dev Technol. 2017;22:889–903.
Abdelbary A, Elshafeey AH, Zidan G. Comparative effects of different cellulosic-based directly compressed orodispersable tablets on oral bioavailability of famotidine. Carbohydr Polym. 2009
K.A. Khalid, Ahmed AH, Mowafaq MG, Alaa A., Elkhodairy KA, Hassan MA, et al. Formulation and optimization of orodispersible tablets of flutamide. Saudi Pharm J. 2014;
Türkmen Ö, Ay Şenyiğit Z, Baloğlu E. Formulation and evaluation of fexofenadine hydrochloride orally disintegrating tablets for pediatric use. J Drug Deliv Sci Technol 2018
Sivannarayana P, Prameela Rani A, Saikishore V. Formulation and in-vitro characterisation of fast disintegrating tablets of ziprosidone. Res J Pharm, Biol Chem Sci. 2013;4:1424–31.
Gülbağ S, Yılmaz Usta D, Gültekin HE, Oktay AN, Demirtaş Ö, Karaküçük A, et al. New perspective to develop memantine orally disintegrating tablet formulations: SeDeM expert system. Pharm Dev Technol. Informa Healthcare USA, Inc; 2018;23:512–9.
Anup N, Thakkar S, Misra M. Formulation of olanzapine nanosuspension based orally disintegrating tablets (ODT); comparative evaluation of lyophilization and electrospraying process as solidification techniques. Adv Powder Technol [Internet]. Society of Powder Technology Japan; 2018;29:1913–24. Available from: https://doi.org/10.1016/j.apt.2018.05.003.
Karakucuk A, Teksin ZS, Eroglu H, Celebi N. Evaluation of improved oral bioavailability of ritonavir nanosuspension. Eur J Pharm Sci [Internet]. Elsevier; 2019;131:153–8. Available from: https://doi.org/10.1016/j.ejps.2019.02.028.
Dolenc A, Kristl J, Baumgartner S, Planinšek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12.
Li F, Li L, Wang S, Yang Y, Li J, Liu D, et al. Improved dissolution and oral absorption by co-grinding active drug probucol and ternary stabilizers mixtures with planetary beads-milling method. Asian J Pharm Sci [Internet]. Elsevier B.V.; 2019;14:649–57. Available from: https://doi.org/10.1016/j.ajps.2018.12.001.
Wang H, Xiao Y, Wang H, Sang Z, Han X, Ren S, et al. Development of daidzein nanosuspensions: preparation, characterization, in vitro evaluation, and pharmacokinetic analysis. Int J Pharm [Internet]. Elsevier; 2019;566:67–76. Available from: https://doi.org/10.1016/j.ijpharm.2019.05.051.
Fernandes AR, Dias-Ferreira J, Cabral C, Garcia ML, Souto EB. Release kinetics and cell viability of ibuprofen nanocrystals produced by melt-emulsification. Colloids Surfaces B Biointerfaces [Internet]. Elsevier B.V.; 2018;166:24–8. Available from: https://doi.org/10.1016/j.colsurfb.2018.03.005.
Shariare MH, Altamimi MA, Marzan AL, Tabassum R, Jahan B, Reza HM, et al. In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using design of experiment (DoE). Saudi Pharm J [Internet]. King Saud University; 2019;27:96–105. Available from: https://doi.org/10.1016/j.jsps.2018.09.002.
Gulsun T, Borna SE, Vural I, Sahin S. Preparation and characterization of furosemide nanosuspensions. J Drug Deliv Sci Technol [Internet]. Elsevier; 2018;45:93–100. Available from: https://doi.org/10.1016/j.jddst.2018.03.005.
Jain S, Reddy VA, Arora S, Patel K. Development of surface stabilized candesartan cilexetil nanocrystals with enhanced dissolution rate, permeation rate across CaCo-2, and oral bioavailability. Drug Deliv Transl Res [Internet]. Drug Delivery and Translational Research; 2016;6:498–510. Available from: https://doi.org/10.1007/s13346-016-0297-8.
Qiao H, Chen L, Rui T, Wang J, Chen T, Fu T, et al. Fabrication and in vitro/in vivo evaluation of amorphous andrographolide nanosuspensions stabilized by D-α-tocopheryl polyethylene glycol 1000 succinate/sodium lauryl sulfate. Int J Nanomedicine. 2017;12:1033–46.
Shekhawat P, Bagul M, Edwankar D, Pokharkar V. Enhanced dissolution/caco-2 permeability, pharmacokinetic and pharmacodynamic performance of re-dispersible eprosartan mesylate nanopowder. Eur J Pharm Sci [Internet]. Elsevier; 2019;132:72–85. Available from: https://doi.org/10.1016/j.ejps.2019.02.021.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev [Internet]. Elsevier B.V.; 2011;63:456–69. Available from: https://doi.org/10.1016/j.addr.2011.02.001.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release [Internet]. Elsevier B.V.; 2013;172:1126–41. Available from: https://doi.org/10.1016/j.jconrel.2013.08.006.
Acknowledgments
The authors would like to thank Abdi Ibrahim for providing ziprasidone hydrochloride monohydrate and Dr. Naile Ozturk for collaborating on cell culture studies.
Funding
This study was supported by a grant from The Scientific and Technological Research Council of Turkey (Project No: 215S920, TUBITAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tashan, E., Karakucuk, A. & Celebi, N. Development of Nanocrystal Ziprasidone Orally Disintegrating Tablets: Optimization by Using Design of Experiment and In Vitro Evaluation. AAPS PharmSciTech 21, 115 (2020). https://doi.org/10.1208/s12249-020-01653-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01653-9