Abstract
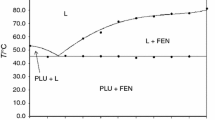
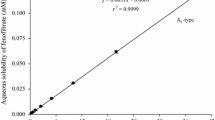
The objective of this study is to explore the surface wettability modulated by a surfactant and its effects on the drug release and absorption of fenofibrate solid dispersions (FF SDs). Both the polyvinylpyrrolidone/sodium lauryl sulfate (PVP/SLS) coprecipitate and FF SDs were prepared by solvent evaporation method. The contact angle of PVP/SLS coprecipitate with various PVP/SLS weight ratios was determined to screen out the suitable content of SLS incorporated in FF SDs. Scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) was used to analyze the surface composition of the PVP/SLS coprecipitate, suggesting that SLS molecules were prone to concentrate on the carrier surface. The physicochemical characteristics of FF, PVP, SLS, FF SDs, and FF physical mixtures (PMs) were evaluated by thermal analysis, XRD, FTIR, and SEM, which revealed that FF was molecularly dispersed in SDs. The interaction between SLS and PVP or FF confirmed by FTIR would affect the surface morphology of SDs. Finally, the contact angle of FF SDs was measured to explore the effects of surface wettability on the dissolution behavior and drug absorption of FF SDs. The interesting thing is that the wettability of the PVP/SLS coprecipitate was positively related to that of FF SDs. The improved wettability of FF SDs or the PVP/SLS coprecipitate by adding SLS contributed to the slight enhancement of initial drug release and absorption, which implied that wettability would be a promising tool in the formulation studies.









Similar content being viewed by others
References
He Y, Ho C. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci Elsevier Masson SAS. 2015;104:3237–58.
Vasconcelos T, Marques S, das Neves J, Sarmento B. Amorphous solid dispersions: rational selection of a manufacturing process. Adv Drug Deliv Rev. 2016;100:85–101.
Chaudhari SP, Dugar RP. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J Drug Deliv Sci Technol Elsevier Ltd. 2017;41:68–77.
Dahlberg C, Millqvist-Fureby A, Schuleit M. Surface composition and contact angle relationships for differently prepared solid dispersions. Eur J Pharm Biopharm. 2008;70:478–85.
Chokshi RJ, Zia H, Sandhu HK, Shah NH, Malick WA. Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution—pros and cons. Drug Deliv. 2007;14:33–45.
Yang B, Wei C, Yang Y, Wang Q, Li S. Evaluation about wettability, water absorption or swelling of excipients through various methods and the correlation between these parameters and tablet disintegration. Drug Dev Ind Pharm. Informa Healthcare USA, Inc. 2018;0:1–9.
Kumar G, Prabhu KN. Review of non-reactive and reactive wetting of liquids on surfaces. Adv Colloid Interf Sci. 2007;133:61–89.
Lu Y, Tang N, Lian R, Qi J, Wu W. Understanding the relationship between wettability and dissolution of solid dispersion. Int J Pharm. 2014;465:25–31.
Li J, Fan N, Wang X, Li C, Sun M, Wang J, et al. Interfacial interaction track of amorphous solid dispersions established by water-soluble polymer and indometacin. Eur J Pharm Sci. Elsevier. 2017;106:244–53.
Li J, Fan N, Li C, Wang J, Li S, He Z. The tracking of interfacial interaction of amorphous solid dispersions formed by water-soluble polymer and nitrendipine. Appl Surf Sci. Elsevier BV. 2017;420:136–44.
Dahlberg C, Fureby A, Schuleit M, Dvinskikh SV, Furó I. Polymer mobilization and drug release during tablet swelling. A 1H NMR and NMR microimaging study. J Control Release. 2007;122:199–205.
Dahlberg C, Millqvist-Fureby A, Schuleit M, Furó I. Polymer-drug interactions and wetting of solid dispersions. Eur J Pharm Sci. 2010;39:125–33.
Jung HJ, Ahn HI, Park JY, Ho MJ, Lee DR, Cho HR, et al. Improved oral absorption of tacrolimus by a solid dispersion with hypromellose and sodium lauryl sulfate. Int J Biol Macromol. Elsevier B.V. 2016;83:282–7.
Mosquera-Giraldo LI, Trasi NS, Taylor LS. Impact of surfactants on the crystal growth of amorphous celecoxib. Int J Pharm. Elsevier B.V. 2014;461:251–7.
Xua S, Dai WG. Drug precipitation inhibitors in supersaturable formulations. Int J Pharm. Elsevier B.V. 2013;453:36–43.
Kim YH, Kim DW, Kwon MS, Cho KH, Kim JO, Yong CS, et al. Clopidogrel napadisilate monohydrate loaded surface-modified solid dispersion: physicochemical characterization and in vivo evaluation. Biol Pharm Bull. 2015;38:1033–40.
Rashid R, Kim DW, Din FU, Mustapha O, Yousaf AM, Park JH, et al. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydr Polym. Elsevier Ltd. 2015;130:26–31.
Bahloul B, Safta F, Lassoued MA, Dhotel H, Seguin J, Mignet N, et al. Use of mouse model in pharmacokinetic studies of poorly water soluble drugs: application to fenofibrate. J Drug Deliv Sci Technol. 2018;43:149–53.
Buch P, Meyer C, Langguth P. Improvement of the wettability and dissolution of fenofibrate compacts by plasma treatment. Int J Pharm. Elsevier B.V. 2011;416:49–54.
Mehmood Yousaf A. Characterization of physicochemical properties of spray-dried solid dispersions loaded with unmodified crystalline fenofibrate. Curr Pharm Anal. 2015;11:139–44.
Goodwin DJ, Picout DR, Ross-Murphy SB, Holland SJ, Martini LG, Lawrence MJ. Ultrasonic degradation for molecular weight reduction of pharmaceutical cellulose ethers. Carbohydr Polym. Elsevier Ltd. 2011;83:843–51.
Company P. Estimation of. Econ Lett. 1982;9:359–64.
Chaudhari SP, Dave RH. Evaluating the effects of different molecular weights of polymers in stabilizing supersaturated drug solutions and formulations using various methodologies of the model drug: fenofibrate. J Pharm Sci Pharmacol. 2015;2:259–76.
Ming-Thau S, Ching-Min Y, Sokoloski TD. Characterization and dissolution of fenofibrate solid dispersion systems. Int J Pharm. 1994;103:137–46.
Pereira MAV, Fonseca GD, Silva-Júnior AA, Fernandes-Pedrosa MF, De M, Barbosa EG, et al. Compatibility study between chitosan and pharmaceutical excipients used in solid dosage forms. J Therm Anal Calorim. 2014;116:1091–100.
Smith LA, Duncan A, Thomson GB, Roberts KJ, Machin D, McLeod G. Crystallisation of sodium dodecyl sulphate from aqueous solution: phase identification, crystal morphology, surface chemistry and kinetic interface roughening. J Cryst Growth. 2004;263:480–90.
Dave RH, Patel HH, Donahue E, Patel AD. To evaluate the change in release from solid dispersion using sodium lauryl sulfate and model drug sulfathiazole. Drug Dev Ind Pharm. 2013;39:1562–72.
Dave RH, Patel AD, Donahue E, Patel HH. To evaluate the effect of addition of an anionic surfactant on solid dispersion using model drug indomethacin. Drug Dev Ind Pharm. 2012;38:930–9.
Yang B, Xu L, Wang Q, Li S. Modulation of the wettability of excipients by surfactant and its impacts on the disintegration and release of tablets. Drug Dev Ind Pharm. 2016;42:1945–55.
Liu T, Hao J, Yang B, Hu B, Cui Z, Li S. Contact angle measurements: an alternative approach towards understanding the mechanism of increased drug dissolution from ethylcellulose tablets containing surfactant and exploring the relationship between their contact angles and dissolution behaviors. AAPS PharmSciTech. 2018;19:1582–91.
Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. 2002;231:131–144.
França MT, Nicolay RP, Klüppel Riekes M, Munari Oliveira Pinto J, Stulzer HK. Investigation of novel supersaturating drug delivery systems of chlorthalidone: the use of polymer-surfactant complex as an effective carrier in solid dispersions. Eur J Pharm Sci. Elsevier. 2018;111:142–52.
Mandal B, Moulik SP, Ghosh S. Influence of aquo-organic solvent media on the self-aggregation of sodium dodecyl sulfate (SDS) and its interaction with polyvinylpyrrolidone (PVP). Colloid Polym Sci. 2014;292:2485–95.
Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJH. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30:2976–92.
Funding
This work was financially supported by the National Science and Technology Major Project (No. 2017ZX09101001-006-012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, B., Wei, C., Qian, F. et al. Surface Wettability Modulated by Surfactant and Its Effects on the Drug Release and Absorption of Fenofibrate Solid Dispersions. AAPS PharmSciTech 20, 234 (2019). https://doi.org/10.1208/s12249-019-1446-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1446-4