Abstract
The aim of the work is to develop a data fusion model using near-infrared (NIR) and process parameters for the predictions of drug dissolution from controlled release multiparticulate beads. Using a design of experiments, ciprofloxacin-coated beads were manufactured and critical process parameters such as air volume, product temperature, curing temperature, and curing time were measured; environmental humidity was monitored using a Pyrobuttons®. The NIR spectra were decomposed using principal component analysis (PCA). The PCA scores were fused with process measurements and all variables were autoscaled. The autoscaled variables were regressed against measured dissolution data at 1 h and 2 h time points; the PLS regression used quadratic and cross terms. The NIR spectra only model using data collected at the end of bead curing generated a PLS model using 5 latent variables with R2 equal to 0.245 and 0.299 and RMSECV 13.23 and 13.12 for the 1 h and 2 h dissolution time points, respectively. The low R2 and high root mean square error of cross validation (RMSECV) values indicate that NIR spectra alone were insufficient to model the drug release. Similar results were obtained for NIR model using data collected at the end of spraying phase. Models with fused spectral and process data yielded better prediction with R2 above 0.88 and RMSECV less than 5% for the 1 h and 2 h dissolution time points. The data fusion model predicted dissolution profiles with an error less than 10%.








Similar content being viewed by others
References
Kothari BH, Fahmy R, Claycamp HG, Moore CMV, Chatterjee S, Hoag SW. A systematic approach of employing quality by design principles: risk assessment and design of experiments to demonstrate process understanding and identify the critical process parameters for coating of the ethylcellulose pseudolatex dispersion using non-conventional fluid bed process. AAPS PharmSciTech 2016:1–23.
De Leersnyder F, Vanhoorne V, Bekaert H, Vercruysse J, Ghijs M, Bostijn N, et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: influence of process parameters and wet granule transfer. Eur J Pharm Sci. 2018;115:223–32.
Mortier STFC, De Beer T, Gernaey KV, Vercruysse J, Fonteyne M, Remon JP, et al. Mechanistic modelling of the drying behaviour of single pharmaceutical granules. Eur J Pharm Biopharm. 2012;80(3):682–9.
Kothari BH, Fahmy R, Claycamp G, Moore C, Chatterjee S, Hoag SW. Comparing a statistical model and Bayesian approach to establish the design space for the coating of ciprofloxacin HCl beads at different scales of production. AAPS PharmsciTech. 2016;19(8):3809–28.
Kothari BH, Fahmy R, Claycamp HG, Moore CMV, Chatterjee S, Hoag SW. A systematic approach of employing quality by design principles: risk assessment and design of experiments to demonstrate process understanding and identify the critical process parameters for coating of the ethylcellulose pseudolatex dispersion using non-conventional fluid bed process. AAPS PharmSciTech. 2016;18(4):1135–57.
Moore CMV. Regulatory perspective on real time release testing (RTRT). AAPS Annual Meeting Washington, DC2011.
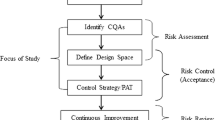
ICH. PHARMACEUTICAL DEVELOPMENT Q8(R2). In: Group IEW, editor. 2009. p. 1–28.
MacGregor JF, Kourti T. Statistical process control of multivariate processes. Control Eng Pract. 1995;3(3):403–14.
Wong CWL, Escott REA, Morris AJ, Martin EB. The integration of process and spectroscopic data for enhanced knowledge extraction in batch processes. In: Puigjaner L, Espuña A, editors. Computer aided chemical engineering. 20: Elsevier; 2005. p. 1141–6.
ICH Harmonised Tripartite Guideline, Pharmaceutical Development, Q8(R2) Revision 2, . 2009.
Ciurczak E. Uses of near-infrared spectroscopy in pharmaceutical analysis. Appl Spectrosc Rev. 1987;23(1–2):147–63.
Freitas MP, Sabadin A, Silva LM, Giannotti FM, do Couto DA, Tonhi E, et al. Prediction of drug dissolution profiles from tablets using NIR diffuse reflectance spectroscopy: a rapid and nondestructive method. J Pharm Biomed Anal. 2005;39(1–2):17–21.
Gabrielsson J, Jonsson H, Airiau C, Schmidt B, Escott R, Trygg J. The OPLS methodology for analysis of multi-block batch process data. J Chemom. 2006;20(8–10):362–9.
Gurden SP, Westerhuis JA, Smilde AK. Monitoring of batch processes using spectroscopy. AICHE J. 2002;48(10):2283–97.
Smilde AK, Westerhuis JA, Boqué R. Multiway multiblock component and covariates regression models. J Chemom. 2000;14(3):301–31.
Tabasi SH, Fahmy R, Bensley D, O’Brien C, Hoag SW. Quality by design, part III: study of curing process of sustained release coated products using NIR spectroscopy. J Pharm Sci. 2008;97(9):4067–86.
Tabasi SH, Fahmy R, Bensley D, O’Brien C, Hoag SW. Quality by design, part II: application of NIR spectroscopy to monitor the coating process for a pharmaceutical sustained release product. J Pharm Sci. 2008;97(9):4052–66.
Tabasi SH, Fahmy R, Bensley D, O’Brien C, Hoag SW. Quality by design, part I: application of NIR spectroscopy to monitor tablet manufacturing process. J Pharm Sci. 2008;97(9):4040–51.
Donoso M, Ghaly ES. Prediction of drug dissolution from tablets using near-infrared diffuse reflectance spectroscopy as a nondestructive method. Pharm Dev Technol. 2004;9(3):247–63.
Tabasi SH, Moolchandani V, Fahmy R, Hoag SW. Sustained release dosage forms dissolution behavior prediction: a study of matrix tablets using NIR spectroscopy. Int J Pharm. 2009;382(1–2):1–6.
Gendre C, Boiret M, Genty M, Chaminade P, Pean JM. Real-time predictions of drug release and end point detection of a coating operation by in-line near infrared measurements. Int J Pharm. 2011;421(2):237–43.
Berglund A, Wold S. INLR, implicit non-linear latent variable regression. J Chemom. 1997;11(2):141–56.
Norgaard L, Saudland A, Wagner J, Nielsen JP, Munck L, Engelsen SB. Interval partial least-squares regression (iPLS): a comparative chemometric study with an example from near-infrared spectroscopy. Appl Spectrosc. 2000;54(3):413–9.
Acknowledgments
We would like to thank Bela Janscik of OPULUS, Inc. for supplying the Pyrobutton® package.
Funding
This project received funding from the U.S. Food and Drug Administration (FDA) under grant no. HHSF223201110076A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: William C. Stagner and Rahul V. Haware
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, A., Kothari, B.H., Fahmy, R. et al. Prediction of Dissolution of Sustained Release Coated Ciprofloxacin Beads Using Near-infrared Spectroscopy and Process Parameters: a Data Fusion Approach. AAPS PharmSciTech 20, 222 (2019). https://doi.org/10.1208/s12249-019-1401-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1401-4