Abstract
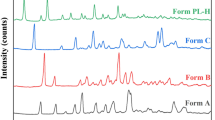
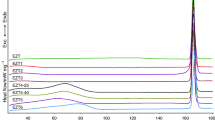
The mechanism of l-Val on how to improve the stability of gabapentin (GBP) was described by the combination of chemical analysis experiments and computer simulations. Scanning electron microscope (SEM), powder X-ray diffraction (PXRD), and differential scanning calorimeter (DSC), coupled with attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), were used to identify β-GBP prepared by rapid solvent removal method. The reaction barriers on crystal planes, β-GBP (100) and β-GBP (10-1), are smaller than α-GBP and γ-GBP, reaching 276.65 kJ/mol and 299.57 kJ/mol, respectively. Thus, it was easier for β-GBP to form lactam, and the occurrence of β-GBP would lead the worse stability of α-GBP. The addition of neutral amino acids such as l-Val could improve the stability of α-GBP effectively. The adsorption energy of α-GBP (002) crystal plane with l-Val is larger than that of other crystal planes, reaching 42.17 kJ/mol. Hydrogen bond was the combination of l-Val and GBP main crystal planes, which could inhibit the crystal transformation of α-GBP. These results suggest that neutral amino acid protectants, such as l-Val, could improve the stability of α-GBP effectively, and inhibition of crystal transformation is one of the effective methods to improve the stability of polymorphic drugs.













Similar content being viewed by others
References
Zong Z, Desai SD, Kaushal AM, Barich DH, Huang H, Munson EJ, et al. The stabilizing effect of moisture on the solid-state degradation of gabapentin. AAPS PharmSciTech. 2011;12(3):924–31.
Herrmann M, Menz J, Olsson O, Kümmerer K. Identification of phototransformation products of the antiepileptic drug gabapentin: biodegradability and initial assessment of toxicity. Water Res. 2015;15(85):11–21.
Kansal S, Sinha P, Agarwa R, Sharma V. Comparison of analgesic efficacy of antiepileptic gabapentin with conventional analgesic diclofenac in rat experimental models. J Drug Deliv Ther. 2017;1(7):44–8.
Singh D, Kennedy DH. The use of gabapentin for the treatment of postherpetic neuralgia. Clin Ther. 2003;25(3):852–89.
Hsu CH, Ke WT, Lin SY. Progressive steps of polymorphic transformation of gabapentin polymorphs studied by hot-stage FTIR microspectroscopy. J Pharm Pharm Sci. 2010;13(1):67–77.
Reddy LS, Bethune SJ, Kampf JW, Rodríguez-Hornedo N. Cocrystals and salts of gabapentin: pH dependent cocrystal stability and solubility. Cryst Growth Des. 2009;1(9):378–85.
Taylora CP, Angelottib T, Faumanc E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2–δ (alpha2–delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73(2):137–50.
Delaney SP, Smith TM, Korter TM. Conformation versus cohesion in the relative stabilities of gabapentin polymorphs. Roval Soc Chem. 2014;4:855–64.
Braga D, Grepioni F, Maini L, Brescello R, Cotarca L. Simple and quantitative mechanochemical preparation of the first zinc and copper complexes of the neuroleptic drug gabapentin. CrystEngComm. 2008;10(5):469–71.
Reece HA, Levendis DC. Polymorphs of gabapentin. Acta Crystallogr C. 2008;64(3):o105–8.
Lin S, Hsu C, Ke W. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int J Pharmaceut. 2010;396(1–2):83–90.
Tinmanee R, Larsen SC, Morris KR, Kirsch LE. Quantification of gabapentin polymorphs in gabapentin/excipient mixtures using solid state 13 C NMR spectroscopy and X-ray powder diffraction. J Pharmaceut Biomed. 2017;146:29–36.
Tinmanee R, Stamatis SD, Ueyama E, Morris KR, Kirsch LE. Polymorphic and covalent transformations of gabapentin in binary excipient mixtures after milling-induced stress. Pharm Res-Dordr. 2018:35–9.
Zong Z, Qiu J, Tinmanee R, Kirsch LE. Kinetic model for solid-state degradation of gabapentin. J Pharm Sci-US. 2012;101(6):2123–33.
Potschka H, Feuerstein TJ, Löscher W. Gabapentin-lactam, a close analogue of the anticonvulsant gabapentin, exerts convulsant activity in amygdala kindled rats. Naunyn Schmiedeberg's Arch Pharmacol. 2000;361(2):200–5.
Jehle T, Feuerstein TJ, Lagrèze WA. The effect of gabapentin and gabapentin-lactam on retinal ganglion cell survival. Situation after acute retinal ischemia in animal models. Europe PMC. 2001;98(3):237–41.
Hsu C, Lin S. Rapid examination of the kinetic process of intramolecular lactamization of gabapentin using DSC–FTIR. Thermochim Acta. 2009;486(1–2):5–10.
Zucker B, Ludin DE, Gerds TA, Lücking CH, Landwehrmeyer GB, Feuerstein TJ. Gabapentin-lactam, but not gabapentin, reduces protein aggregates and improves motor performance in a transgenic mouse model of Huntington's disease. Naunyn Schmiedeberg's Arch Pharmacol. 2004;270(2):131–9.
SH Q, invento Stable pharmaceutical preparation of γ-aminobutyric acid derivative and preparation method. 1999 1999-05-10.
Chakravarty P, Lubach JW, Hau J, Nagapudi K. A rational approach towards development of amorphous solid dispersions: experimental and computational techniques. Int J Pharmaceut. 2017;519(1–2):44–57.
Zhao Q, Miriyala N, Su Y, Chen W, Gao X, Shao L, et al. Computer-aided formulation design for a highly soluble lutein–cyclodextrin multiple-component delivery system. Mol Pharmaceut. 2018;15(4):1664–73.
Ouyang D, Smith SC. Application of molecular modeling in drug delivery. Computational Pharmaceutics. Hoboken: Wiley; 2015. p. 1–4.
Ramezanpour M, Leung SSW, Delgado-Magnero KH, Bashe BYM, Thewalt J, Tieleman DP. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim Biophys Acta Biomembr. 2016;1858(7):1688–709.
Ouyang D. Investigating the molecular structures of solid dispersions by the simulated annealing method. Chem Phys Lett. 2012;554:177–84.
Chen W, Ouyang D. Investigation of molecular dissolution mechanism of ketoprofen binary and ternary solid dispersions by molecular dynamics simulations. Mol Simul. 2017;43:1074–80.
Zhang Q, Jiang L, Mei X. Thermodynamic and kinetic investigation of agomelatine polymorph transformation. Pharm Dev Technol. 2016;2(21):196–203.
Frisch M J TGWS. Gaussian 09. Revision D.02. 2009.
Li G, Wang D, Huang Z. Crystalline interface phase study. J Synth Cryst. 2001;2(30):171–7.
Liu XY, Boek ES, Briels WJ. Prediction of crystal growth morphology based on structural analysis of the solid–fluid interface. Nature. 1995;6520(374):342–5.
Liu N, Zhou C, Shu Y, Wang B, Wang W. Molecular dynamics study on crystal morphology of N-mercaptourea dinitramide salt. Chem J Chin Univ. 2017;(12):2231–7.
André V, Fernandes A, Santos PP, Duarte MT. On the track of new multicomponent gabapentin crystal forms: synthon competition and pH stability. Cryst Growth Des. 2011;6(11):2325–34.
Jain D, Mishra M, Rani A. Synthesis and characterization of novel aminopropylated fly ash catalyst and its beneficial application in base catalyzed Knoevenagel condensation reaction. Fuel Process Technol. 2012;95:119–26.
Hamied Y, Kankan R, Rao D, ^inventors; Polymorphic forms of olanzapine. 2002 2002-02-19.
Wildfong PLD, Morley NA, Moore MD, Morris KR. Quantitative determination of polymorphic composition in intact compacts by parallel-beam X-ray powder diffractometry II-data correction for analysis of phase transformations as a function of pressure. J Pharmaceut Biomed. 2005;39(1–2):1–7.
Achrai B, Libster D, Aserin A, Garti N. Solubilization of gabapentin into HII mesophases. J Phys Chem. 2010;115(5):825–35.
Dong Z, Munson EJ, Schroeder SA, Prakash I, Grant DJW. Neotame anhydrate polymorphs II-quantitation and relative physical stability. Pharm Res-Dordr. 2002;19(9):1259–64.
Schammé B, Couvrat N, Malpeli P, Dudognon E, Delbreilh L, Dupray V, et al. Transformation of an active pharmaceutical ingredient upon high-energy milling—a process-induced disorder in Biclotymol. Int J Pharmaceut. 2016;499(1–2):67–73.
Calvo NL, Kaufman TS, Maggio RM. A PCA-based chemometrics-assisted ATR-FTIR approach for the classification of polymorphs of cimetidine: application to physical mixtures and tablets. J Pharmaceut Biomed. 2015;107(25):419–25.
Volpe DA, Gupta A, Ciavarella AB, Faustino PJ, Sayeed VA, Khan MA. Comparison of the stability of split and intact gabapentin tablets. Int J Pharmaceut. 2008;350(1–2):65–9.
Zhang J, Lv X. Study on the stability of gabapentin. Journal of Chemical Engineering of Chinese Universities. 2012 2012-10-15(05):800–5.
Sun H, Ren P, Fried JR. The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci. 1998;1–2(8):229–46.
Bunte SW. Molecular modeling of energetic materials: the parameterization and validation of nitrate esters in the COMPASS force field. J Phys Chem B. 2000;11(104):235–65.
Ibers JA. Gabapentin and gabapentin monohydrate. Acta Cryst. 2001;C57:641–3.
Tokmakoff A, Lang MJ, Jordanides XJ, Fleming GR. The intermolecular interaction mechanisms in liquid CS2 at 295 and 165 K probed with two-dimensional Raman spectroscopy. Chem Phys. 1998;233(2):231–42.
Mogi I, Kamiko M. Striking effects of magnetic field on the growth morphology of electrochemical deposits. J Cryst Growth. 1996;166(1–4):276–80.
Braga D, Grepioni F, Maini L, Rubini K, Polito M, Brescello R, et al. Polymorphic gabapentin: thermal behaviour, reactivity and interconversion of forms in solution and solid-state. New J Chem. 2008;10:1645–808.
Ehtezazi T, Govender T, Stolnik S. Hydrogen bonding and electrostatic interaction contributions to the interaction of a cationic drug with polyaspartic acid. Pharm Res-Dordr. 2000;17(7):871–8.
Funding
This work was supported by Shanghai Science and Technology Commission R&D Platform Special (18DZ2290500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimers
The views expressed in the manuscript entitled “Investigating The Mechanism of L-Valine In Improving The Stability of Gabapentin Combining Chemical Analysis Experiments With Computational Pharmacy,” which we wish to be considered for publication in “AAPS PharmSciTech,” are my own and not an official position of the institution or funder.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Yang, L., Li, D. et al. Investigating the Mechanism of l-Valine in Improving the Stability of Gabapentin Combining Chemical Analysis Experiments with Computational Pharmacy. AAPS PharmSciTech 20, 114 (2019). https://doi.org/10.1208/s12249-019-1312-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1312-4