Abstract
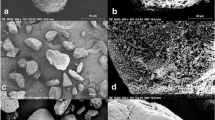
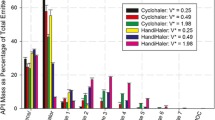
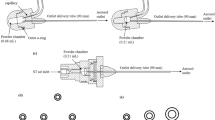
This study aims to investigate the implications of loaded formulation mass on aerosol performance using a reservoir novel dry powder inhaler containing a custom dosing cup to deliver carrier-based formulation to the lungs. A 3D printed dosing cup with volume size of 133.04 mm3 was manufactured to allow for the progressive loading of different carrier formulation masses of 1% beclomethasone dipropionate BDP (w/w) formulation (10 to 60 mg, with increments of 10 mg), in a novel customizable DPI device. Scanning electron micrographs were used to investigate BDP detachment from carrier particles post-aerosolisation and particle deposition on the USP induction port. The subsequent aerosol performance analysis was performed using the next generation impactor (NGI). Incrementally increasing the loading mass to 60 mg led to decreases in BDP detachment from carrier particles, resulting in significant decreases in aerosol performance. Increases in loading dose mass led to progressively decreased detachment of BDP from the carrier and the overall aerosol performance in comparison to the initial mass of 10 mg. These results are likely to be due to a decrease in void volume within the dosing cup with increased loading mass leading to altered airflow, decreased impaction forces and the possibility of a significant quantity of large carrier particles introducing a ‘sweeping’ effect on the inhaler inner surface. This study has shown that despite the decreased BDP detachment from the carrier and decreased aerosol performance, the dose delivered to the lung still increased due to the higher loaded dose.








Similar content being viewed by others
References
Claus S, Weiler C, Schiewe J, Friess W. How can we bring high drug doses to the lung? Eur J Pharm Biopharm. 2014;86(1):1–6.
Yeung S, Traini D, Tweedie A, Lewis D, Church T, Young PM. Limitations of high dose carrier based formulations. Int J Pharm. 2018;544(1):141–52.
Schwarz C. Colobreathe® for the treatment of cystic fibrosis-associated pulmonary infections. Pulm Ther. 2015;1(1):19–30.
Vandevanter DR, Geller DE. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: a review. Med Devices. 2011;4:179–88.
Nakano T, Ishiwada N, Sumitani T, Uemori M, Isobe K, Laninamivir Prophylaxis Study G. Inhaled laninamivir octanoate as prophylaxis for influenza in children. Pediatrics. 2016;138(6).
Daiichi-Sankyo-Company-Limited. Inavir-Instructions of use 2018:2.
Begat P, Morton DAV, Staniforth JN, Price R. The cohesive-adhesive balances in dry powder inhaler formulations I: direct quantification by atomic force microscopy. Pharm Res. 2004;21(9):1591–7.
Parumasivam T, Leung SS, Tang P, Mauro C, Britton W, Chan HK. The delivery of high-dose dry powder antibiotics by a low-cost generic inhaler. AAPS J. 2017;19(1):191–202.
Farkas DR, Hindle M, Longest PW. Characterization of a new high-dose dry powder inhaler (DPI) based on a fluidized bed design. Ann Biomed Eng. 2015;43(11):2804–15.
Hoppentocht M, Akkerman OW, Hagedoorn P, Frijlink HW, de Boer AH. The Cyclops for pulmonary delivery of aminoglycosides; a new member of the Twincer family. Eur J Pharm Biopharm 2015;90:8–15.
Yang Y, Yang Z, Ren Y, Mei X. Effects of formulation and operating variables on zanamivir dry powder inhalation characteristics and aerosolization performance. Drug Deliv. 2014;21(6):480–6.
Young PM, Crapper J, Philips G, Sharma K, Chan HK, Traini D. Overcoming dose limitations using the orbital® multi-breath dry powder inhaler. J Aerosol Med pulmonary Drug Deliv. 2014;27(2):138–47.
Zhu B, Padroni M, Colombo G, Phillips G, Crapper J, Young PM, et al. The development of a single-use, capsule-free multi-breath tobramycin dry powder inhaler for the treatment of cystic fibrosis. Int J Pharm. 2016;514(2):392–8.
Maltz DS, Paboojian J. Device engineering insights into TOBI Podhaler: a development case study of high efficiency powder delivery to cystic fibrosis patients. Respir Drug Deliv. 2011;1:55–66.
Schuster A, Haliburn C, Doring G, Goldman MH, Freedom Study G. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax. 2013;68(4):344–50.
Hagedoorn P, Grasmeijer F, Hoppentocht M, Akkerman O, Frijlink H, De Boer A. In vitro evaluation of the Twincer colistin dry powder inhaler as a non-cough-inducing alternative to Colobreathe. Eur Respir J. 2016;48(Suppl 60):PA2561.
Fernandes JV, deAndrade GR, Villax P. Scaling up for high dose delivery to the lungs. ONdrugDelivery. 2016;(72):30–3.
de Boer AH, Hagedoorn P, Woolhouse R, Wynn E. Computational fluid dynamics (CFD) assisted performance evaluation of the Twincer disposable high-dose dry powder inhaler. J Pharm Pharmacol. 2012;64(9):1316–25.
GlaxoSmithKline. Highlights of prescribing information - Incruse® Ellipta®. 2016.
Buttini F, Brambilla G, Copelli D, Sisti V, Balducci AG, Bettini R, et al. Effect of flow rate on in vitro aerodynamic performance of NEXThaler in comparison with Diskus and Turbohaler dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2015;28:1–12.
Corradi M, Chrystyn H, Cosio BG, Pirozynski M, Loukides S, Louis R, et al. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11(9):1497–506.
British Pharmacopoeia Commision. British Pharmacopoeia Volume V Appendix XII C. Consistency of Formulated Preparations 3. Uniformity of Content. London: TSO; 2017.
British Pharmacopoeia Commision. British Pharmacopoeia Volume V Appendix XII C. Consistency of Formulated Preparations 7. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles. London: TSO; 2017.
Shalash AO, Elsayed MMA. A new role of fine excipient materials in carrier-based dry powder inhalation mixtures: effect on deagglomeration of drug particles during mixing revealed. AAPS PharmSciTech. 2017;18(8):2862–70.
Young PM, Edge S, Traini D, Jones MD, Price R, El-Sabawi D, et al. The influence of dose on the performance of dry powder inhalation systems. Int J Pharm. 2005;296(1–2):26–33.
Grasmeijer F, Lexmond AJ, van den Noort M, Hagedoorn P, Hickey AJ, Frijlink HW, et al. New mechanisms to explain the effects of added lactose fines on the dispersion performance of adhesive mixtures for inhalation. PLoS One. 2014;9(1):e87825.
de Boer AH, Dickhoff BH, Hagedoorn P, Gjaltema D, Goede J, Lambregts D, et al. A critical evaluation of the relevant parameters for drug redispersion from adhesive mixtures during inhalation. Int J Pharm. 2005;294(1–2):173–84.
Tang P, Kwok PC, Tong Z, Yang R, Raper JA, Chan HK. Does the United States Pharmacopeia throat introduce de-agglomeration of carrier-free powder from inhalers? Pharm Res. 2012;29(7):1797–807.
Peng T, Lin S, Niu B, Wang X, Huang Y, Zhang X, et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm Sin B. 2016;6(4):308–18.
Yang RY, Zou RP, Yu AB. Computer simulation of the packing of fine particles. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;62(3 pt B):3900–8.
Yang RY, Zou RP, Yu AB, Choi SK. Characterization of interparticle forces in the packing of cohesive fine particles. Phys Rev E Stat Nonlinear Soft Matter Phys. 2008;78(3 Pt 1):031302.
Garcia A, Mack P, Williams S, Fromen C, Shen T, Tully J, et al. Microfabricated engineered particle systems for respiratory drug delivery and other pharmaceutical applications. J Drug Deliv. 2012;2012:941243.
Ariane M, Sommerfeld M, Alexiadis A. Wall collision and drug-carrier detachment in dry powder inhalers: using DEM to devise a sub-scale model for CFD calculations. Powder Technol. 2018;334:65–75.
de Boer AH, Hagedoorn P, Gjaltema D, Goede J, Frijlink H. Air classifier technology (ACT) in dry powder inhalationPart 1. Introduction of a novel force distribution concept (FDC) explaining the performance of a basic air classifier on adhesive mixtures. Int J Pharm. 2003;260(2):187–200.
de Boer AH, Hagedoorn P, Westerman EM, Le Brun PP, Heijerman HG, Frijlink HW. Design and in vitro performance testing of multiple air classifier technology in a new disposable inhaler concept (Twincer) for high powder doses. Eur J Pharm Sci. 2006;28(3):171–8.
Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195–203.
Behara SR, Farkas DR, Hindle M, Longest PW. Development of a high efficiency dry powder inhaler: effects of capsule chamber design and inhaler surface modifications. Pharm Res. 2014;31(2):360–72.
Capece M, Silva KR, Sunkara D, Strong J, Gao P. On the relationship of inter-particle cohesiveness and bulk powder behavior: Flowability of pharmaceutical powders. Int J Pharm. 2016;511(1):178–89.
Weiler C, Wolkenhauer M, Trunk M, Langguth P. New model describing the total dispersion of dry powder agglomerates. Powder Technol. 2010;203(2):248–53.
Acknowledgements
Australian Research Council ARC LP120200744.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Philip J. Kuehl and Stephen W. Stein
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yeung, S., Traini, D., Tweedie, A. et al. Assessing Aerosol Performance of a Dry Powder Carrier Formulation with Increasing Doses Using a Novel Inhaler. AAPS PharmSciTech 20, 94 (2019). https://doi.org/10.1208/s12249-019-1302-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1302-6