Abstract
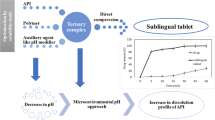
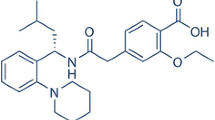
The aim of this research was to design and evaluate a hydrophilic matrix system for sustained release of glipizide, a weakly acidic poor soluble drug. A combination of inclusion complexation and microenvironmental pH modification techniques was utilized to improve the dissolution and pH-independent release of glipizide. Hydroxypropyl-β-cyclodextrin (HP-β-CD) was used as the complexation agent while sodium citrate and magnesium oxide (MgO) were used as model pH modifiers. The hydrophilic matrix tablets were prepared by powder direct compression and evaluated by in vitro dissolution study respectively in pH 6.8 and pH 1.2 dissolution media. The formulations containing MgO exhibited increased cumulative drug release from less than 40% in the reference formulation to 90% within 24 h in acidic media (pH 1.2). The release profile in acidic media was similar to the alkaline media (pH 6.8) with a similarity factor (f2) of 55.0, suggesting the weakening of the effect of pH on the dissolution efficiency of glipizide. The release profile fitted well into the Higuchi model and the dominant mechanism of drug release was Fickian diffusion while case II transport/polymer relaxation occurred. In conclusion, combining inclusion complexation agents and pH modifiers had improved the dissolution of glipizide as well as achieved the pH-independent release profile.









Similar content being viewed by others
References
Taniguchi C, Kawabata Y, Wada K, et al. Microenvironmental pH-modification to improve dissolution behavior and oral absorption for drugs with pH-dependent solubility. Expert Opin Drug Deliv. 2014;11:505–16.
Pang J, Dalziel G, Dean B, et al. Pharmacokinetics and absorption of the anticancer agents dasatinib and GDC-0941 under various gastric conditions in dogs—reversing the effect of elevated gastric pH with betaine HCl. Mol Pharm. 2013;10:4024–31.
McConnell EL, Fadda HM, Basit AW. Gut instincts: explorations in intestinal physiology and drug delivery. Int J Pharm. 2008;364:213–26.
Mitra A, Kesisoglou F. Impaired drug absorption due to high stomach pH: a review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol Pharm. 2013;10:3970–9.
Varum FJ, Hatton GB, Basit AW. Food, physiology and drug delivery. Int J Pharm. 2013;457:446–60.
Merchant HA, Goyanes A, Parashar N, et al. Predicting the gastrointestinal behaviour of modified-release products: utility of a novel dynamic dissolution test apparatus involving the use of bicarbonate buffers. Int J Pharm. 2014;475:585–91.
McAllister M. Dynamic dissolution: a step closer to predictive dissolution testing? Mol Pharm. 2010;7:1374–87.
Bassi P, Kaur G. pH modulation: a mechanism to obtain pH-independent drug release. Expert Opin Drug Deliv. 2010;7:845–57.
Taniguchi C, Inoue R, Kawabata Y, et al. Novel formulations of dipyridamole with microenvironmental pH-modifiers for improved dissolution and bioavailability under hypochlorhydria. Int J Pharm. 2012;434:148–54.
Rao VM, Engh K, Qiu Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int J Pharm. 2003;252:81–6.
Riis T, Bauer-Brandl A, Wagner T, et al. pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. Eur J Pharm Biopharm. 2007;65:78–84.
Tran PH, Tran HT, Lee BJ. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J Control Release. 2008;129:59–65.
Adachi M, Hinatsu Y, Kusamori K, et al. Improved dissolution and absorption of ketoconazole in the presence of organic acids as pH-modifiers. Eur J Pharm Sci. 2015;76:225–30.
Onoue S, Inoue R, Taniguchi C, et al. Improved dissolution and pharmacokinetic behavior of dipyridamole formulation with microenvironmental pH-modifier under hypochlorhydria. Int J Pharm. 2012;426:61–6.
Hamza Yel S, Aburahma MH. Design and in vitro evaluation of novel sustained-release matrix tablets for lornoxicam based on the combination of hydrophilic matrix formers and basic pH-modifiers. Pharm Dev Technol. 2010;15:139–53.
Zur M, Cohen N, Agbaria R, et al. The biopharmaceutics of successful controlled release drug product: segmental-dependent permeability of glipizide vs. metoprolol throughout the intestinal tract. Int J Pharm. 2015;489:304–10.
Kivistö KT, Neuvonen PJ. Enhancement of absorption and effect of glipizide by magnesium hydroxide. Clin Pharmacol Ther. 1991;49:39–43.
Nguyen C, Christensen JM, Ayres JW. Compression of coated drug beads for sustained release tablet of glipizide: formulation, and dissolution. Pharm Dev Technol. 2014;19:10–20.
Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm. 2004;57:513–25.
Huang H, Wu Z, Qi X, et al. Compression-coated tablets of glipizide using hydroxypropylcellulose for zero-order release: in vitro and in vivo evaluation. Int J Pharm. 2013;446:211–8.
Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154:2–19.
Cuzzucoli Crucitti V, Maria Migneco L, et al. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur J Pharm Biopharm. 2018;125:114–23.
Baird JA, Taylor LS. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64:396–421.
Wang D, Chen G, Ren L. Preparation and characterization of the sulfobutylether-beta-cyclodextrin inclusion complex of amiodarone hydrochloride with enhanced oral bioavailability in fasted state. AAPS PharmSciTech. 2017;18:1526–35.
Patel R, Purohit N. Physico-chemical characterization and in vitro dissolution assessment of clonazepam-cyclodextrins inclusion compounds. AAPS PharmSciTech. 2009;10:1301–12.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug–excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Ezawa T, Inoue Y, Murata I, et al. Characterization of the dissolution behavior of piperine/cyclodextrins inclusion complex. APPS PharmSciTech. 2018;19:923–33.
Kawabata Y, Wada K, Nakatani M, et al. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420:1–10.
Nair AB, Attimarad M, Al-Dhubiab BE, et al. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-beta-cyclodextrin. Drug Deliv. 2014;21:540–7.
Tang P, Li S, Wang L, et al. Inclusion complexes of chlorzoxazone with β- and hydroxypropyl-β-cyclodextrin: characterization, dissolution, and cytotoxicity. Carbohydr Polym. 2015;131:297–305.
Taneri F, Ozcan I, Guneri T. In vitro and in vivo evaluation of oral tablet formulations prepared with ketoconazole and hydroxypropyl-β-cyclodextrin. Drug Deliv. 2010;17:152–7.
Pygall SR, Kujawinski S, Timmins P, et al. The suitability of tris(hydroxylmethyl) aminomethane (THAM) as a buffering system for hydroxypropyl methylcellulose (HPMC) hydrophilic matrices containing a weak acid drug. Int J Pharm. 2010;387:93–102.
Menning MM, Dalziel SM. Fumaric acid microenvironment tablet formulation and process development for crystalline cenicriviroc mesylate, a BCS IV compound. Mol Pharm. 2013;10:4005–15.
Tran PH, Tran TT, Park JB, et al. Investigation of physicochemical factors affecting the stability of a pH-modulated solid dispersion and a tablet during storage. Int J Pharm. 2011;414:48–55.
Vyslouzil J, Pavlokova S, Vetchy D. Influence of pH modulation on dynamic behavior of gel layer and release of weakly basic drug from HPMC/wax matrices, controlled by acidic modifiers evaluated by multivariate data analysis. AAPS PharmSciTech. 2017;18:1242–53.
Varma MV, Kaushal AM, Garg S. Influence of micro-environmental pH on the gel layer behavior and release of a basic drug from various hydrophilic matrices. J Control Release. 2005;103:499–510.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48:139–57.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2012;64:163–74.
Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57:169–72.
Funding
This research was supported by Funds of Shenzhen Science and Technology Innovation Commission (Program No. JCYJ20160428091243299).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Huang, J., Lin, H., Peng, B. et al. Design and Evaluation of Hydrophilic Matrix System for pH-Independent Sustained Release of Weakly Acidic Poorly Soluble Drug. AAPS PharmSciTech 19, 2144–2154 (2018). https://doi.org/10.1208/s12249-018-1008-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1008-1