Abstract
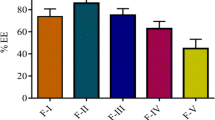
The aim of this study was to develop a proliposomal formulation of lipopeptide antibiotic drug daptomycin (DAP) for oral delivery. Thin film hydration was the selected method for preparation of proliposomes. Different phospholipids including soy-phosphatidylcholine (SPC), hydrogenated egg-phosphatidylcholine (HEPC), and distearoyl-phosphatidylcholine (DSPC) were evaluated in combination with cholesterol. The inclusion of surface charge modifiers in the formulation such as dicetyl phosphate (DCP) and stearylamine (SA) to enhance drug encapsulation was also evaluated. Particle size, surface charge, and encapsulation efficiency were performed on daptomycin-hydrated proliposomes as part of physical characterization. USP type II dissolution apparatus with phosphate buffer (pH 6.8) was used for in vitro drug release studies. Optimized formulation was evaluated for in vivo pharmacokinetics after oral administration to Sprague-Dawley rats. Proliposomes composed of SPC exhibited higher entrapment efficiency than those containing HEPC or DSPC. The highest entrapment efficiency was achieved by positively charged SPC-SA proliposomes, showing an encapsulation efficiency of 92% and a zeta potential of + 28 mV. In vitro drug release of optimized formulation demonstrated efficient drug retention totaling for less than 20% drug release within the first 60 min and only 42% drug release after 2 h. Pharmacokinetic parameters after single oral administration of optimized proliposomal formulation indicated a significant increase in oral bioavailability of DAP administered as SPC-SA proliposomes when compared to drug solution. Based on these results, incorporation of charge modifiers into proliposomes may increase drug loading and proliposomes an attractive carrier for oral delivery of daptomycin.


Similar content being viewed by others
References
Singh R, Singh S, Lillard JW. Past, present, and future technologies for oral delivery of therapeutic proteins. J Pharm Sci. 2008;97(7):2497–523. https://doi.org/10.1002/jps.21183.
Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447(1–2):75–93. https://doi.org/10.1016/j.ijpharm.2013.02.030.
Gupta S, Jain A, Chakraborty M, Sahni JK, Ali J, Dang S. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Deliv. 2013;20(6):237–46.
Ismail R, Csóka I. Novel strategies in the oral delivery of antidiabetic peptide drugs—insulin, GLP 1 and its analogs. Eur J Pharm Biopharm. 2017;115:257–67. https://doi.org/10.1016/j.ejpb.2017.03.015.
Gupta V, Hwang BH, Doshi N, Mitragotri S. A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine. J Control Release. 2013;172(2):541–9. https://doi.org/10.1016/j.jconrel.2013.05.002.
Bernkop-Schnürch A. Reprint of: nanocarrier systems for oral drug delivery: do we really need them? Eur J Pharm Sci. 2013;50(1):2–7. https://doi.org/10.1016/j.ejps.2013.06.011.
Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. https://doi.org/10.1016/j.jsps.2014.06.004.
Payne NI, Timmins P, Ambrose CV, Ward MD, Ridgway F. Proliposomes: a novel solution to an old problem. J Pharm Sci. 1986;75(4):325–9.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Potluri P, Betageri GV. Mixed-micellar proliposomal systems for enhanced oral delivery of progesterone. Drug Deliv. 2006;13(3):227–32. https://doi.org/10.1080/10717540500395007.
Nekkanti V, Venkatesan N, Betageri G. Proliposomes for oral delivery: progress and challenges. Curr Pharm Biotechnol. 2015;16:303–12.
Wang JP, Maitani Y, Takayama K, Pharmacokinetics NT. Antitumor effect of doxorubicin carried by stealth and remote loading proliposome. Pharm Res. 2000;17(7):782–7. https://doi.org/10.1023/a:1007543805947.
Song KH, Chung SJ, Shim CK. Preparation and evaluation of proliposomes containing salmon calcitonin. J Controll Release: Off J Controll Release Soc. 2002;84(1–2):27–37.
Murata M, Yonamine T, Tanaka S, Tahara K, Tozuka Y, Takeuchi H. Surface modification of liposomes using polymer–wheat germ agglutinin conjugates to improve the absorption of peptide drugs by pulmonary administration. J Pharm Sci. 2013;102(4):1281–9. https://doi.org/10.1002/jps.23463.
Colletier J-P, Chaize B, Winterhalter M, Fournier D. Protein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002;2(1):9. https://doi.org/10.1186/1472-6750-2-9.
Veerapu G, Gangadharappa HV, Nagashubha B, Balamuralidhara V. Review on novel carrier system: liposomes and proliposomes. Drug Deliv Lett. 2014;4(2):96–109.
Tantisripreecha C, Jaturanpinyo M, Panyarachun B, Sarisuta N. Development of delayed-release proliposomes tablets for oral protein drug delivery. Drug Dev Ind Pharm. 2012;38(6):718–27. https://doi.org/10.3109/03639045.2011.623168.
Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother. 2003;47(4):1318–23.
Zaffiri L, Gardner J, Toledo-Pereyra LH. History of antibiotics: from fluoroquinolones to daptomycin (part 2). J Investig Surg. 2013;26(4):167–79. https://doi.org/10.3109/08941939.2013.808461.
Zupančič O, Partenhauser A, Lam HT, Rohrer J, Bernkop-Schnürch A. Development and in vitro characterisation of an oral self-emulsifying delivery system for daptomycin. Eur J Pharm Sci. 2016;81:129–36. https://doi.org/10.1016/j.ejps.2015.10.005.
Hiremath PS, Soppimath KS, Betageri GV. Proliposomes of exemestane for improved oral delivery: formulation and in vitro evaluation using PAMPA, Caco-2 and rat intestine. Int J Pharm. 2009;380(1–2):96–104. https://doi.org/10.1016/j.ijpharm.2009.07.008.
Yanamandra S, Venkatesan N, Kadajji VG, Wang Z, Issar M, Betageri GV. Proliposomes as a drug delivery system to decrease the hepatic first-pass metabolism: case study using a model drug. Eur J Pharm Sci. 2014;64:26–36. https://doi.org/10.1016/j.ejps.2014.08.008.
Tobin CM, Darville JM, Lovering AM, MacGowan AP. An HPLC assay for daptomycin in serum. J Antimicrob Chemother. 2008;62(6):1462–3. https://doi.org/10.1093/jac/dkn414.
Nekkanti V, Venkatesan N, Wang Z, Betageri GV. Improved oral bioavailability of valsartan using proliposomes: design, characterization and in vivo pharmacokinetics. Drug Dev Ind Pharm. 2015;41(12):2077–88. https://doi.org/10.3109/03639045.2015.1075026.
Nekkanti V, Rueda J, Wang Z, Betageri GV. Comparative evaluation of proliposomes and self micro-emulsifying drug delivery system for improved oral bioavailability of nisoldipine. Int J Pharm. 2016;505(1–2):79–88. https://doi.org/10.1016/j.ijpharm.2016.03.065.
Betageri GV. Liposomal encapsulation and stability of dideoxyinosine triphosphate. Drug Dev Ind Pharm. 1993;19(5):531–9. https://doi.org/10.3109/03639049309062965.
El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308(1–2):140–8. https://doi.org/10.1016/j.ijpharm.2005.11.006.
Zschörnig O, Arnold K, Richter W, Ohki S. Dextran sulfate-dependent fusion of liposomes containing cationic stearylamine. Chem Phys Lipids. 1992;63(1):15–22. https://doi.org/10.1016/0009-3084(92)90016-I.
Sułkowski WW, Pentak D, Nowak K, Sułkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J Mol Struct. 2005;744–747:737–47. https://doi.org/10.1016/j.molstruc.2004.11.075.
Khoo SM, Shackleford DM, Porter CJH, Edwards GA, Charman WN. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. 2003.
Kulkarni S, Betageri G, Singh M. Factors affecting microencapsulation of drugs in liposomes. J Microencapsul. 1995;12(3):229–46.
Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OO. Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv. 2011;18(8):599–612. https://doi.org/10.3109/10717544.2011.604686.
Grit M, Crommelin DJA. Chemical stability of liposomes: implications for their physical stability. Chem Phys Lipids. 1993;64(1):3–18. https://doi.org/10.1016/0009-3084(93)90053-6.
Sakoulas G, Eliopoulos GM, Alder J, Eliopoulos CT. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47(5):1714–8.
Acknowledgements
The authors express their sincere gratitude to Western University of Health Sciences, Pomona, California for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Arregui, J.R., Kovvasu, S.P. & Betageri, G.V. Daptomycin Proliposomes for Oral Delivery: Formulation, Characterization, and In Vivo Pharmacokinetics. AAPS PharmSciTech 19, 1802–1809 (2018). https://doi.org/10.1208/s12249-018-0989-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-0989-0