Abstract
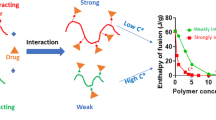
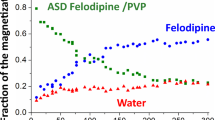
The solvent-shift method was used to identify appropriate polymers that inhibit the growth of felodipine crystals by monitoring particle size in supersaturated drug solutions in the presence of different polymers. We speculated that there would be an intermolecular interaction between the selected polymer (zein) and felodipine by extrapolating the inhibitory effect on crystal growth and then used the selected polymer as a carrier to prepare solid dispersions. The formulations were characterized by crystalline properties, thermodynamics of mixing, dissolution behavior, and physical stability. Powder x-ray diffraction and differential scanning calorimetry experiments indicated that amorphous solid dispersions were formed when the proportion of felodipine was < 30% (w/w). Stability tests showed that a solid dispersion with 20% felodipine remained in an amorphous state and was stable under accelerated storage conditions for 6 months. The dissolution rates of solid dispersions were significantly greater than those of the active pharmaceutical ingredient or physical mixtures. Analysis by Fourier-transform infrared spectroscopy and Raman microspectroscopy indicated the formation of intermolecular interactions between zein and felodipine. The study demonstrates the successful application of the chosen polymer as a carrier in solid dispersions and validates the concept of extrapolating the inhibitory effect on crystal growth to intermolecular interactions.












Similar content being viewed by others
References
Sarode AL, Malekar SA, Cote C, Worthen DR. Hydroxypropyl cellulose stabilizes amorphous solid dispersions of the poorly water soluble drug felodipine. Carbohydr Polym. 2014;112C(21):512–9.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. https://doi.org/10.1016/S0939-6411(00)00076-X.
Loung CW, Sidney R. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60(9):1281–302.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–51. https://doi.org/10.1007/s11095-008-9721-1.
Karavas E, Ktistis G, Xenakis A, Georgarakis E. Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Eur J Pharm Biopharm. 2006;63(2):103–14. https://doi.org/10.1016/j.ejpb.2006.01.016.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9. https://doi.org/10.1016/j.ejpb.2008.05.023.
Kestur US, Taylor LS. Role of polymer chemistry in influencing crystal growth rates from amorphous felodipine. CrystEngComm. 2010;12(8):2390–7. https://doi.org/10.1039/c001905d.
Lu J, Shah S, Jo S, Majumdar S, Gryczke A, Kolter K, et al. Investigation of phase diagrams and physical stability of drug–polymer solid dispersions. Pharm Dev Technol. 2015;20(1):105–17. https://doi.org/10.3109/10837450.2014.949269.
Yamashita T, Ozaki S, Kushida I. Solvent shift method for anti-precipitant screening of poorly soluble drugs using biorelevant medium and dimethyl sulfoxide. Int J Pharm. 2011;419(1–2):170–4. https://doi.org/10.1016/j.ijpharm.2011.07.045.
Sun M, Wu C, Qiang F, Di D, Kuang X, Wang C, et al. Solvent-shift strategy to identify suitable polymers to inhibit humidity-induced solid-state crystallization of lacidipine amorphous solid dispersions. Int J Pharm. 2016;503(1–2):238–46. https://doi.org/10.1016/j.ijpharm.2016.01.062.
Konno H, Taylor LS. Ability of different polymers to inhibit the crystallization of amorphous felodipine in the presence of moisture. Pharm Res. 2008;25(4):969–78. https://doi.org/10.1007/s11095-007-9331-3.
Wu C, Zhao Z, Zhao Y, Hao Y, Liu Y, Liu C. Preparation of a push–pull osmotic pump of felodipine solubilized by mesoporous silica nanoparticles with a core–shell structure. Int J Pharm. 2014;475(1–2):298–305. https://doi.org/10.1016/j.ijpharm.2014.08.033.
Kim EJ, Chun MK, Jang JS, Lee IH, Lee KR, Choia HK. Preparation of a solid dispersion of felodipine using a solvent wetting method. Eur J Pharm Biopharm. 2006;64(2):200–5. https://doi.org/10.1016/j.ejpb.2006.04.001.
Lu J, Cuellar K, Hammer NI, Jo S, Gryczke A, Kolter K, et al. Solid-state characterization of felodipine-soluplus amorphous solid dispersions. Drug Dev Ind Pharm. 2016;42(3):485–96. https://doi.org/10.3109/03639045.2015.1104347.
Wan ZL, Guo J, Yang XQ. Plant protein-based delivery systems for bioactive ingredients in foods. Food Funct. 2015;6(9):2876–89. https://doi.org/10.1039/C5FO00050E.
Qin D, Zhang L, Du X, Wang Y, Zhang Q. Simple and green synthesis of protein-conjugated CdS nanoparticles and spectroscopic study on the interaction between CdS and zein. J Nanopart Res. 2016;18(9):254–64. https://doi.org/10.1007/s11051-016-3568-x.
Kaushik S, Pathak K. Solvent wetting method—a novel approach for preparation of felodipine solid dispersion. Int J Pharm Sci Lett. 2012;2(6):163–6.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42.
Mishra A, Maiti P. Aromatic polyurethanes: the effect of hard segment and chain structure on their properties. J Polym Eng. 2011;31(2–3):253–9. https://doi.org/10.1515/polyeng.2011.051.
Olabisi O, Robeson LM, Shaw MT. Polymer–polymer miscibility. New York: Academic Press; 1979.
Donnelly C, Tian Y, Potter C, Jones DS, Andrews GP. Probing the effects of experimental conditions on the character of drug–polymer phase diagrams constructed using Flory–Huggins theory. Pharm Res. 2014;32(1):167–79. https://doi.org/10.1007/s11095-014-1453-9.
Rubinstein M, Marsac PJ. Polymer physics. New York: Oxford University Press; 2003.
Iskandar M, Krause S. Phase separation in styrene-a-methyl styrene block copolymers. Polym alloys Polym Sci Technol. 1978;10:231–43.
Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm Res. 1995;12(6):799–806. https://doi.org/10.1023/A:1016292416526.
Vasanthavada M, Tong WQ, Joshi Y, Kislalioglu MS. Phase behavior of amorphous molecular dispersions II: role of hydrogen bonding in solid solubility and phase separation kinetics. Pharm Res. 2005;22(3):440–8. https://doi.org/10.1007/s11095-004-1882-y.
Mahmah O, Tabbakh R, Kelly A, Paradkar AA. Comparative study of the effect of spray drying and hot-melt extrusion on the properties of amorphous solid dispersions containing felodipine. J Pharm Pharmacol. 2014;66(2):275–84. https://doi.org/10.1111/jphp.12099.
Tung NT, Hung MV, Vo XM, Nguyen TH, Pham TMH. Formulation optimization of orally disintegrating tablets containing solid dispersion of felodipine and hydroxypropyl methylcellulose using face-centered central composite design. J Pharm Investig. 2014;44(2):111–8. https://doi.org/10.1007/s40005-013-0106-z.
Sun C, Liu F, Yang J, Yang W, Yuan F, Gao Y. Physical, structural, thermal and morphological characteristics of zeinquercetagetin composite colloidal nanoparticles. Ind Crop Prod. 2015;77:476–83. https://doi.org/10.1016/j.indcrop.2015.09.028.
Hsu BL, Weng YM, Liao YH, Chen W. Structural investigation of edible zein films/coatings and directly determining their thickness by FT-Raman spectroscopy. J Agric Food Chem. 2005;53(13):5089–95. https://doi.org/10.1021/jf0501490.
Acknowledgments
This work was supported by the Baoshan District Committee of Science and Technology (grant no. bkw2014131) and the National Natural Science Foundation of China (grant no. 21576080).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of Interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Fu, J., Cui, L., Yang, C. et al. Screen for Inhibitors of Crystal Growth to Identify Desirable Carriers for Amorphous Solid Dispersions Containing Felodipine. AAPS PharmSciTech 19, 1231–1242 (2018). https://doi.org/10.1208/s12249-017-0942-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0942-7