Abstract
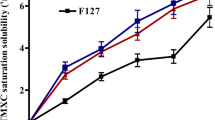
Raloxifene (RLX) has been strongly recommended for postmenopausal women at high risk of invasive breast cancer and for prevention of osteoporosis. However, low aqueous solubility and reduced bioavailability hinder its clinical application. The objective of this study was to explore the potential of RLX loaded mixed micelles (RLX-MM) using Pluronic F68 and Gelucire 44/14 for enhanced bioavailability and improved anticancer activity on human breast cancer cell line (MCF-7). RLX-MM were prepared by solvent evaporation method and optimized using 32 factorial design. The average size, entrapment efficiency and zeta potential of the optimized formulation were found to be 190 ± 3.3 nm, 79 ± 1.3%, 13 ± 0.8 mV, respectively. In vitro study demonstrated 74.68% drug release from RLX-MM in comparison to 42.49% drug release from RLX dispersion. According to the in vitro cytotoxicity assay, GI50 values on MCF-7 breast cancer cell line for RLX-MM and free RLX were found to be 22.5 and 94.71 μg/mL, respectively. Significant improvement (P < 0.05) in the anticancer activity on MCF-7 cell line was observed in RLX-MM over RLX pure drug. Additionally, oral bioavailability of RLX-MM was improved by 1.5-fold over free RLX when administered in female Wistar rats. Incorporation of RLX in the hydrophobic core and improved solubility of the drug due to hydrophilic shell attributed to the enhanced cytotoxicity and bioavailability of RLX-MM. This research establishes the potential of RLX loaded mixed micelles of Pluronic F68 and Gelucire 44/14 for improved bioavailability and anticancer activity on MCF-7 cell line.








Similar content being viewed by others
References
Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15(19/20):842–50. https://doi.org/10.1016/j.drudis.2010.08.006.
Michels KB. Risk of breast cancer with hormone replacement therapy. Int Congr Ser. 2002;1129:135–41.
Miller E, Lee HJ, Lulla A, Hernandez L, Gokare P, Lim B. Current treatment of early breast cancer: adjuvant and neoadjuvant therapy. F1000Res. 2014;3:198. 10.12688/f1000research.4340.1.
Ağardan NBM, Değim Z, et al. The effectiveness of raloxifene-loaded liposomes and cochleates in breast cancer therapy. AAPS PharmSciTech. 2015;17(4):968–77. https://doi.org/10.1208/s12249-015-0429-3.
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of Raloxifene. J Natl Cancer Inst. 2004;96(23):1751–61. https://doi.org/10.1093/jnci/djh319.
Mizuma T. Intestinal glucuronidation metabolism may have a greater impact on oral bioavailability than hepatic glucuronidation metabolism in humans: a study with raloxifene, substrate for UGT1A1, 1A8, 1A9, and 1A10. Int J Pharm. 2009;378(1–2):140–1. https://doi.org/10.1016/j.ijpharm.2009.05.044.
Tran T, Poudel B, et al. Preparation and evaluation of raloxifene loaded solid dispersion nanoparticle by spray drying without an organic solvent. Int J Pharm. 2013;443(1-2):50–7. https://doi.org/10.1016/j.ijpharm.2013.01.013.
Thakkar H, Nangesh J, Parmar M, Patel D. Formulation and characterisation of lipid based drug delivery systems of raloxifene—microemulsions and self-microemulsifying drug delivery system. J Pharm Bioallied Sci. 2011;3(3):442–8. https://doi.org/10.4103/0975-7406.84463.
Jagadish B, Yelchuri R, Bindu K, Tangi H, Maroju S, Rao VU. Enhanced dissolution and bioavailability of RLX hydrochloride by co-grinding with different superdisintegrants. Chem Pharm Bull. 2010;58(3):293–300. https://doi.org/10.1248/cpb.58.293.
Michael FW, Wacher VJ, Ruble KM, et al. Pharmacokinetics of RLX in male Wistar–Hannover rats: influence of complexation with hydroxybutenyl-beta-cyclodextrin. Int J Pharm. 2008;346:25–37.
Rfsler A, Vandermeulen GM, Klok H-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev. 2001;53(1):95–108. https://doi.org/10.1016/S0169-409X(01)00222-8.
Xiong X-B, Falamarzian A, Garg SM, Lavasanifar A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J Control Release. 2011;155(2):248–61. https://doi.org/10.1016/j.jconrel.2011.04.028.
Gao Z-G, Fain HD, Rapoport N. Controlled and targeted tumor therapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102(1):203–22. https://doi.org/10.1016/j.jconrel.2004.09.021.
Saxena V, Delwar M. Poloxamer 407/TPGS mixed micelles for delivery of gam-bogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713–21.
Bothiraja C, Kapre H, et al. Development of plumbagin-loaded phospholipid–Tween 80 mixed micelles: formulation, optimization, effect on breast cancer cells and human blood/serum compatibility testing. Ther Deliv. 2013;4(10):1247–59. https://doi.org/10.4155/tde.13.92.
Sezgin Z, Yuksel N, Baykara T. Preparation and characterization of polymeric micelles for solubilisation of poorly soluble anticancer drugs. Eur J Pharm Biopharm. 2006;64(3):261–8. https://doi.org/10.1016/j.ejpb.2006.06.003.
Kuo JH. Effect of Pluronic-block copolymers on the reduction of serum-mediated inhibition of gene transfer of polyethyleneimine-DNA complexes. Biotechnol Appl Biochem. 2003;37(Pt 3):267–71. https://doi.org/10.1042/BA20020123.
BASF, The Chemical Company. http://worldaccount.basf.com/wa/NAFTA/Catalog/ChemicalsNAFTA/doc4/BASF/PRD/30089194/.pdf?asset_type=pi/pdf&language=EN&urn=urn:documentum:eCommerce_sol_EU:09007bb28001f73c.pdf. Accessed - 26 Nov 2015.
Liu Z, Liu D, Wang L, Zhang J, Zhang N. Docetaxel-loaded Pluronic P123 polymeric micelles: in vitro and in vivo evaluation. Int J Mol Sci. 2011;12(12):1684–96. https://doi.org/10.3390/ijms12031684.
Singh V, Khullar P, Dave P, Kaur N. Micelles, mixed micelles, and applications of polyoxypropylene (PPO)-polyoxyethylene (PEO)-polyoxypropylene (PPO) triblock polymers. Int J Ind Chem. 2013;4(1):12. https://doi.org/10.1186/2228-5547-4-12.
Zhang J, Li Y, Fang X, Zhou D, Wang Y, Chen M. TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int J Pharm. 2014;476(1-2):185–98. https://doi.org/10.1016/j.ijpharm.2014.09.017.
Mehanny M, Hathout R, et al. Bisdemethoxycurcumin loaded polymeric mixed micelles as potential anti-cancer remedy: preparation, optimization and cytotoxic evaluation in a HepG-2 cell model. J Mol Liq. 2016;214:162–70. https://doi.org/10.1016/j.molliq.2015.12.007.
Patel HR, Patel RP, Patel MM. Poloxamers: a pharmaceutical excipients with therapeutic behaviours. Int J PharmTech Res. 2009;1(2):299–303.
Patel P, Mehta T, Panchal S. Preparation, evaluation and comparison of lipid based drug delivery systems of tacrolimus. Int J Pharm Pharm Sci. 2014;6(2):588–91.
Upadhyay P, Kumar J, Kumar A. Gelucire: an alternative formulation technological tool for both sustained and fast release of drug in treating disbetes milletus type-II disease. J Sci Ind Res. 2013;72:776–80.
Kawakami K, Miyoshi K, Ida Y. Solubilization behaviour of poorly soluble drugs with combined use of Gelucire 44/14 and cosolvent. J Pharm Sci. 2004;93(6):1471–9. https://doi.org/10.1002/jps.20067.
Sato M. RLX: selective estrogen receptor modulator. J Bone Miner Metab. 1994;12(2):S9–S20. https://doi.org/10.1007/BF02383389.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1-2):176–85. https://doi.org/10.1016/j.ijpharm.2009.04.030.
Patil S, Choudhary B, Rathore A, Roy K, Mahadik K. Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine. 2015;22(12):1103–11. https://doi.org/10.1016/j.phymed.2015.08.006.
Fisher R. Introduction to “The arrangement of field experiments”. J Minist Agric G B. 1926;33:503–13.
Kuchekar A, Pawar A. Screening of factors using plackett burman design in the preparation of capecitabine loaded nano polymeric micelles. Int J Pharm Pharm Sci. 2014;6(5):489–96.
Suneetha D, Lakshmana Rao A. A new validated RP-HPLC method for the estimation of RLX in pure and tablet dosage form. Rasayan J Chem. 2010;3(1):117–21.
Mourya VK, Nazma I, Nawale RB, Kulthe SS. Polymeric micelles: general considerations and their applications. Ind J Pharm Edu Res. 2011;45(2):128–38.
Li J, Jiang Y, Wen J, Fan G, Wu Y, Zhang C. A rapid and simple HPLC method for the determination of curcumin in rat plasma: assay development, validation and application to pharmacokinetic study of curcumin liposome. Biomed Chromatogr. 2009;23(11):1201–7. https://doi.org/10.1002/bmc.1244.
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65. https://doi.org/10.1016/j.nantod.2012.01.002.
Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf B: Biointerfaces. 2015;130:23–30. https://doi.org/10.1016/j.colsurfb.2015.03.059.
Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–89. https://doi.org/10.1007/s11095-010-0233-4.
Mandpe L, Pokharkar V. Targeted brain delivery of Iloperidone nanostructured lipid carriers following intranasal administration: in vivo pharmacokinetics and brain distribution studies. J Nanopharm Drug Deliv. 2013;1:1–14.
Muller RH, Maeder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. https://doi.org/10.1016/S0939-6411(00)00087-4.
Jha RK, Tiwari S, Mishra B. Bioadhesive microspheres for bioavailability enhancement of RLX hydrochloride: formulation and pharmacokinetic evaluation. Am Assoc Pharm Sci. 2011;12(2):650–7.
Costa P, Sausa J. Modelling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33. https://doi.org/10.1016/S0928-0987(01)00095-1.
Ravi PR, Aditya N, Kathuria H, Malekar S, Vats R. N lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in-vivo evaluation and uptake mechanism. Eur J Pharm Biopharm. 2014;87(1):114–24. https://doi.org/10.1016/j.ejpb.2013.12.015.
Kushwaha AK, Vuddanda PR, Karunanidhi P, Singh SK, Singh S. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. Hindawi Publishing Corporation. Biomed Res Int. 2013;2013:1–9. https://doi.org/10.1155/2013/584549.
Acknowledgments
The authors thank Zim Laboratories (Nagpur, India) and Novartis Pharmaceuticals (Hyderabad, India) for providing us gift samples of raloxifene and carbamazepine, respectively. The authors are much obliged to Alembic Pharmaceuticals (Mumbai, India) and Gattefossé S.A.S. (Saint Priest, France) for providing Pluronic F68 and GL44 gift samples, respectively. Radhika Kanade would also like to thank AICTE (New Delhi, India) for providing financial support during the entire research study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Kanade, R., Boche, M. & Pokharkar, V. Self-Assembling Raloxifene Loaded Mixed Micelles: Formulation Optimization, In Vitro Cytotoxicity and In Vivo Pharmacokinetics. AAPS PharmSciTech 19, 1105–1115 (2018). https://doi.org/10.1208/s12249-017-0919-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0919-6