Abstract
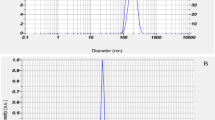
Lacidipine (LCDP) is a highly lipophilic calcium channel blocker of poor aqueous solubility leading to poor oral absorption. This study aims to prepare and optimize LCDP nanosuspensions using antisolvent sonoprecipitation technique to enhance the solubility and dissolution of LCDP. A three-factor, three-level Box–Behnken design was employed to optimize the formulation variables to obtain LCDP nanosuspension of small and uniform particle size. Formulation variables were as follows: stabilizer to drug ratio (A), sodium deoxycholate percentage (B), and sonication time (C). LCDP nanosuspensions were assessed for particle size, zeta potential, and polydispersity index. The formula with the highest desirability (0.969) was chosen as the optimized formula. The values of the formulation variables (A, B, and C) in the optimized nanosuspension were 1.5, 100%, and 8 min, respectively. Optimal LCDP nanosuspension had particle size (PS) of 273.21 nm, zeta potential (ZP) of −32.68 mV and polydispersity index (PDI) of 0.098. LCDP nanosuspension was characterized using x-ray powder diffraction, differential scanning calorimetry, and transmission electron microscopy. LCDP nanosuspension showed saturation solubility 70 times that of raw LCDP in addition to significantly enhanced dissolution rate due to particle size reduction and decreased crystallinity. These results suggest that the optimized LCDP nanosuspension could be promising to improve oral absorption of LCDP.












Similar content being viewed by others
References
Patravale V, Dandekar P, Jain R. Nanoparticulate systems as drug carriers: the need. In: Nanoparticulate drug delivery. 1st ed: Woodhead Publishing; 2012. p. 1–28.
Shegokar R, Muller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399(1–2):129–39.
Kesisoglou F, Mitra A. Crystalline nanosuspensions as potential toxicology and clinical oral formulations for BCS II/IV compounds. AAPS J. 2012;14(4):677–87.
Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68(2):283–8.
Seedher N, Kanojia M. Micellar solubilization of some poorly soluble antidiabetic drugs: a technical note. AAPS PharmSciTech. 2008;9(2):431–6.
Seedher N, Bhatia S. Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech. 2003;4(3):E33.
Sinha S, Ali M, Baboota S, Ahuja A, Kumar A, Ali J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech. 2010;11(2):518–27.
Ran Y, Zhao L, Xu Q, Yalkowsky SH. Solubilization of cyclosporin A. AAPS PharmSciTech. 2001;2(1):E2.
Cao F, Guo J, Ping Q. The physicochemical characteristics of freeze-dried scutellarin-cyclodextrin tetracomponent complexes. Drug Dev Ind Pharm. 2005;31(8):747–56.
Manna L, Banchero M, Sola D, Ferri A, Ronchetti S, Sicardi S. Impregnation of PVP microparticles with ketoprofen in the presence of supercritical CO2. J Supercrit Fluids. 2007;42(3):378–84.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30.
Sinha B, Muller RH, Moschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–41.
Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23.
Junghanns JU, Muller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–309.
Langguth P, Hanafy A, Frenzel D, Grenier P, Nhamias A, Ohlig T, et al. Nanosuspension formulations for low-soluble drugs: pharmacokinetic evaluation using spironolactone as model compound. Drug Dev Ind Pharm. 2005;31(3):319–29.
Jacobs C, Muller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19(2):189–94.
Moschwitzer J, Achleitner G, Pomper H, Muller RH. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58(3):615–9.
Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18(2):113–20.
Liversidge GG, Conzentino P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int J Pharm. 1995;125:309–13.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63(6):427–40.
Lai F, Pini E, Angioni G, Manca ML, Perricci J, Sinico C, et al. Nanocrystals as tool to improve piroxicam dissolution rate in novel orally disintegrating tablets. Eur J Pharm Biopharm. 2011;79(3):552–8.
Mitri K, Shegokar R, Gohla S, Anselmi C, Muller RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420(1):141–6.
Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415(1–2):293–300.
Xia D, Quan P, Piao H, Sun S, Yin Y, Cui F. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40(4):325–34.
Liu D, Xu H, Tian B, Yuan K, Pan H, Ma S, et al. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation-ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech. 2012;13(1):295–304.
Montgomery DC. Design and analysis of experiments. 5th ed. New York: Wiley; 2001.
Ferreira SL, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, et al. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–86.
Solanki AB, Parikh JR, Parikh RH. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level Box-Behnken design. AAPS PharmSciTech. 2007;8(4):E86.
Hanes DS, Weir MR. Calcium antagonists. In: Battegay EJ, Lip GYH, Bakris GL, editors. Hypertension: principles and practice. USA: Taylor & Francis Group; 2005. p. 517–30.
ElKasabgy NA, Elsayed I, Elshafeey AH. Design of lipotomes as a novel dual functioning nanocarrier for bioavailability enhancement of lacidipine: in-vitro and in-vivo characterization. Int J Pharm. 2014;472(1–2):369–79.
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391(1–2):203–11.
Gannu R, Palem CR, Yamsani VV, Yamsani SK, Yamsani MR. Enhanced bioavailability of lacidipine via microemulsion based transdermal gels: formulation optimization, ex vivo and in vivo characterization. Int J Pharm. 2010;388(1–2):231–41.
Zhao J, Luo L, Fu Q, Guo B, Li Y, Geng Y, et al. Improved oral bioavailability of lacidipine using nanosuspension technology: inferior in vitro dissolution and superior in vivo drug absorption verus lacipil(R). Curr Drug Deliv. 2015; in press.
Darekar T, Aithal KS, Shirodkar R, Kumar L, Attari Z, Lewis S. Characterization and in vivo evaluation of lacidipine inclusion complexes with β-cyclodextrin and its derivatives. J Incl Phenom Macrocycl Chem. 2016;84(3):225–35.
Wu C, Kou L, Ma P, Gao L, Li B, Li R, et al. Interspecies prediction of oral pharmacokinetics of different lacidipine formulations from dogs to human: physiologically based pharmacokinetic modelling combined with biorelevant dissolution. RSC Adv. 2015;5:19844–52.
Soliman SM, Abdelmalak NS, El-Gazayerly ON, Abdelaziz N. Novel non-ionic surfactant proniosomes for transdermal delivery of lacidipine: optimization using 2(3) factorial design and in vivo evaluation in rabbits. Drug Deliv. 2016;23(5):1608–22.
Sultana S, Bhavna, Iqbal Z, Panda BP, Talegaonkar S, Bhatnagar A, et al. Lacidipine encapsulated gastroretentive microspheres prepared by chemical denaturation for pylorospasm. J Microencapsul. 2009;26(5):385–93.
Mukharya A, Chaudhary S, Mansuri N, Misra AK. Solid-state characterization of lacidipine/PVP K(29/32) solid dispersion primed by solvent co-evaporation. Int J Pharm Investig. 2012;2(2):90–6.
Sun M, Wu C, Fu Q, Di D, Kuang X, Wang C, et al. Solvent-shift strategy to identify suitable polymers to inhibit humidity-induced solid-state crystallization of lacidipine amorphous solid dispersions. Int J Pharm. 2016;503(1–2):238–46.
Shirodkar R, Reddy N, Kumar N, Ramalingayya GV, Mutalik S, Lewis S. Subacute toxicity study of lacidipine nanoformulation. Lat Am J Pharm. 2015;34(8):1526–33.
Anuradha K, Kumar MS. Development of lacidipine loaded nanostructured lipid carriers (NLCs) for bioavailability enhancement. Int J Pharm Med Res. 2014;2(2):50–7.
Dinda SC, Pand SK. Formulation and in-vitro/in-vivo assessment of enhanced bioavailability of lacidipine using Nanopure technique. Albanian J Pharm Sci. 2014;1:20–5.
Abdelbary G, Makhlouf A. Adoption of polymeric micelles to enhance the oral bioavailability of dexibuprofen: formulation, in-vitro evaluation and in-vivo pharmacokinetic study in healthy human volunteers. Pharm Dev Technol. 2014;19(6):717–27.
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, et al. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using Box-Behnken design. AAPS PharmSciTech. 2015;16(1):118–28.
Tenjarla S, Puranajoti P, Kasina R, Mandal T. Preparation, characterization, and evaluation of miconazole-cyclodextrin complexes for improved oral and topical delivery. J Pharm Sci. 1998;87(4):425–9.
Gonnissen Y, Goncalves SI, De Geest BG, Remon JP, Vervaet C. Process design applied to optimise a directly compressible powder produced via a continuous manufacturing process. Eur J Pharm Biopharm. 2008;68(3):760–70.
Deng J, Huang L, Liu F. Understanding the structure and stability of paclitaxel nanocrystals. Int J Pharm. 2010;390(2):242–9.
Wang N, Hsu C, Zhu L, Tseng S, Hsu JP. Influence of metal oxide nanoparticles concentration on their zeta potential. J Colloid Interface Sci. 2013;407:22–8.
Shah B, Kakumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci. 2006;95(8):1641–65.
Mauludin R, Muller RH, Keck CM. Development of an oral rutin nanocrystal formulation. Int J Pharm. 2009;370(1–2):202–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Claudio Salomon, Francisco Goycoolea, and Bruno Moerschbacher
Rights and permissions
About this article
Cite this article
Kassem, M.A.A., ElMeshad, A.N. & Fares, A.R. Enhanced Solubility and Dissolution Rate of Lacidipine Nanosuspension: Formulation Via Antisolvent Sonoprecipitation Technique and Optimization Using Box–Behnken Design. AAPS PharmSciTech 18, 983–996 (2017). https://doi.org/10.1208/s12249-016-0604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0604-1