Abstract
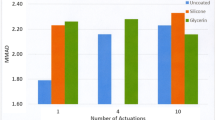
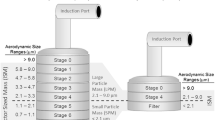
The performance of two quality control (QC) tests for aerodynamic particle size distributions (APSD) of orally inhaled drug products (OIPs) is compared. One of the tests is based on the fine particle dose (FPD) metric currently expected by the European regulators. The other test, called efficient data analysis (EDA), uses the ratio of large particle mass to small particle mass (LPM/SPM), along with impactor sized mass (ISM), to detect changes in APSD for QC purposes. The comparison is based on analysis of APSD data from four products (two different pressurized metered dose inhalers (MDIs) and two dry powder inhalers (DPIs)). It is demonstrated that in each case, EDA is able to detect shifts and abnormalities that FPD misses. The lack of sensitivity on the part of FPD is due to its “aggregate” nature, since FPD is a univariate measure of all particles less than about 5 μm aerodynamic diameter, and shifts or changes within the range encompassed by this metric may go undetected. EDA is thus shown to be superior to FPD for routine control of OIP quality. This finding augments previously reported superiority of EDA compared with impactor stage groupings (favored by US regulators) for incorrect rejections (type I errors) when incorrect acceptances (type II errors) were adjusted to the same probability for both approaches. EDA is therefore proposed as a method of choice for routine quality control of OIPs in both European and US regulatory environments.











Similar content being viewed by others
References
US Food and Drug Administration (FDA), CDER (1998) Draft guidance for industry: metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products; chemistry, manufacturing, and controls Documentation, FDA.http://www.fda.gov/ucm/groups/fdagov-public/documents/document/ucm070573.pdf. Accessed 26 Aug 2015
European Medicines Agency (EMA) (2006) Guideline on the pharmaceutical quality of inhalation and nasal products. EMEA/CHMP/QWP/49313/2005 Final. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf. Accessed 26 Aug 2015
Health Canada (HC) (2006) Therapeutic Products Directorate. Guidance for Industry - Pharmaceutical Quality of Inhalation and Nasal Products. TPD File 06-106624-547. http://hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/chem/inhalationnas-eng.php. Accessed 26 Aug 2015
Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, et al. Next generation pharmaceutical impactor. Part 1: Design. J Aerosol Med. 2003;16(3):283–99.
European Directorate for Quality in Medicines (EDQM) (2014) European pharmacopeia 8.0, monograph 2.9.18. Preparations for inhalations: aerodynamic assessment of fine particles. Strasburg, France EDQM
Tougas TP, Christopher D, Mitchell JP, Strickland H, Wyka B, Van Oort M, et al. Improved quality control metrics for cascade impaction measurements of orally inhaled drug products (OIPs). AAPS PharmSciTech. 2009;10(4):1276–85.
European Directorate for Quality in Medicines (EDQM) (2014) European pharmacopeia 8.0, monograph 2.9.44. Preparations for nebulization. Strasburg, France EDQM
Tougas T, Mitchell J, Lyapustina S. Editors, good cascade impactor practices, AIM and EDA for orally inhaled products. Springer. 2013. doi:10.1007/978-1-4614-6296-5.
Tougas TP, Christopher D, Mitchell J, Lyapustina S, Van Oort M, Bauer R, et al. Product lifecycle approach to cascade impaction measurements. AAPS PharmSciTech. 2011;12(1):312–22. doi:10.1208/s12249-011-9590-5.
Mitchell J, Tougas T, Christopher D, Bauer R, Church T, Dey M, et al. Abbreviated impactor measurement (AIM) and efficient data analysis (EDA) concepts: a partnership for the assurance of oral inhaled product (OIP) quality. Inhalation. 2010;4(4):6–10.
Tougas T, Efficient data analysis in quality assessment, In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, editors. Respiratory Drug Delivery Europe-2011, River Grove, IL: Davis Healthcare Int. Publ; pp.209-214 on-line at http://www.rddonline.com/publications/articles/article.php?ArticleID=1591&return=1. Accessed 26 Aug 2015
Mitchell JP, Nagel MW, Doyle CC, Ali RS, Avvakoumova VI, Cristopher JD, et al. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen cascade impactors (ACIs): Part 1. AAPS PharmSciTech. 2010;11(2):843–51. doi:10.1208/s12249-010-9452-6.
Mitchell JP, Nagel MW, Doyle CC, Ali RS, Avvakoumova VI, Cristopher JD, et al. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen cascade impactors (ACIs): part 2—investigation of bias in extra-fine mass fraction with AIM-HRT impactor. AAPS PharmSciTech. 2010;11(3):1115–8. doi:10.1208/s12249-010-9473-1.
Mitchell J, Christopher D, Tougas T, Glaab V, Lyapustina S. When could efficient data analysis (EDA) fail? Theoretical considerations. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, editors. Respiratory Drug Delivery Europe-2011, River Grove, IL: Davis Healthcare Int. Publ; pp 237–246. http://www.rddonline.com/publications/articles/article.php?ArticleID=1594&return=1
Mitchell J, Tougas T, Lyapustina S. The abbreviated impactor measurement (AIM) and efficient data analysis (EDA) concepts: why they are important and how to work with them. Inhalation. 2012;6(60):13–9.
Crompton GK. How to achieve good compliance with inhaled asthma therapy. Respir Med. 2004;98:S35–40.
Lavorini F (2015) The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy 2013; Article ID 102418, available at: http://dx.doi.org/10.1155/2013/102418
Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, Broedersh M, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604.
Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16(4):341–77.
Sandell D, Tougas T. Quality considerations in the establishment of specifications for pharmaceuticals. Stat Biopharm Res. 2012;4(2):125–35.
Mitchell JP, Dunbar C. The interpretation of data from cascade impactors. J Aerosol Med. 2005;18(4):439–51.
Christopher JD, Strickland H, Morgan B, Dey M, Silcock A, Tougas TP, Mitchell JP, Lyapustina SA (2013) Performance characteristics of EDA and its potential to improve decision making in product batch release. In: Tougas T, Mitchell J, Lyapustina S, Editors, Good Cascade Impactor Practices, AIM and EDA for Orally Inhaled Products, Springer, pp. 173–249. DOI 10.1007/978-1-4614-6296-5
Description of IPAC-RS database: http://www.fda.gov/ohrms/dockets/ac/00/techrepro/3609_rpt2.pdf
Christopher JD, Dey M, Lyapustina S, Mitchell JP, Tougas TP, Van Oort M, et al. Generalized simplified approaches for MMAD determination. Pharm Forumul. 2010;36(3):812–23.
US Food and Drug Administration (FDA), CDER (2002) Guidance for industry: Nasal spray and inhalation solution, suspension, and spray drug products; chemistry, manufacturing, and controls documentation, FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070575.pdf. Accessed 26 Aug 2015
Mitchell JP, Nagel MW, Nichols S, Nerbrink O. Laser diffractometry as a technique for the rapid assessment of aerosol particle size from inhalers. J Aerosol Med. 2006;19(4):409–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tougas, T.P., Goodey, A.P., Hardwell, G. et al. A Comparison of the Performance of Efficient Data Analysis Versus Fine Particle Dose as Metrics for the Quality Control of Aerodynamic Particle Size Distributions of Orally Inhaled Pharmaceuticals. AAPS PharmSciTech 18, 451–461 (2017). https://doi.org/10.1208/s12249-016-0508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0508-0