Abstract
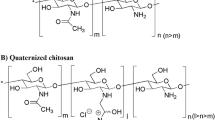
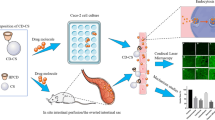
The aim of this study was to investigate the effects of a type of hydrophobic moiety, extent of N-substitution (ES), and degree of quaternization (DQ) of chitosan (CS) on the transepithelial electrical resistance and permeability of Caco-2 cells monolayer, using fluorescein isothiocyanate dextran 4,400 (FD-4) as the model compound for paracellular tight junction transport. CS was substituted with hydrophobic moiety, an aliphatic aldehyde (n-octyl) or aromatic aldehyde (benzyl), for the improved hydrophobic interaction with cell membrane, and they were quaternized with Quat-188 to render CS soluble. The factors affecting the epithelial permeability have been evaluated in the intestinal cell monolayers, Caco-2 cells. Cytotoxicity was evaluated by using the trypan blue and MTT viability assay. The results revealed that at pH 7.4 CSQ appeared to increase cell permeability in dose-dependent manner, and this effect was relatively reversible at the lower doses of 0.05–1.25 mM. The higher DQ and ES caused the higher permeability of FD-4. Cytotoxicity of CSQ was concentration, %DQ, and %ES dependent. Substitution with hydrophobic moiety caused decreasing in permeability of FD-4 and cytotoxicity by benzyl group had more effect than octyl group. These studies demonstrated that these novel modified chitosan derivatives had potential for using as absorption enhancers.









Similar content being viewed by others
Abbreviations
- BzCSQ:
-
N-Benzyl chitosan Quat-188
- CS:
-
Chitosan
- CSA:
-
Chitosan acetate
- CSQ:
-
Chitosan Quat-188
- DQ:
-
Degree of Quat-188
- ES:
-
The extent of N-substitution
- FD-4:
-
Fluorescein isothiocyanate dextran 4,400
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- OctCSQ:
-
N-Octyl chitosan Quat-188
- TEER:
-
The transepithelial electrical resistance
- TM-Bz-CS:
-
Methylated N-(4-N,N-dimethylaminobenzyl) chitosan
References
Borchard G, Lueszen HL, Boer DAG, Verhoef JC, Lehr CM, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption III. Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Rel. 1996;39:131–8.
Portero A, Remunan-Lopez C, Nielsen HM. The potential of chitosan in enhancing peptide and protein absorption across the TR146 cell culture model-an in vitro model of the buccal epithelium. Pharm Res. 2002;19:169–74.
Colo DG, Burgalassi S, Zambito Y, Monti D, Chetoni P. Effects of different N-trimethylchitosans on in vitro/in vivo ofloxacin transcorneal permeation. J Pharm Sci. 2004;93:2851–62.
Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci. 2004;21:351–9.
Sieval AB, Thanou M, Kotze AF, Verhoef JC, Brussee J, Junginger HE. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr Polym. 1998;36:157–65.
Hamman JH, Kotze AF. Effect of the type of base and number of reaction steps on the degree of quaternization and molecular weight of N-trimethyl chitosan chloride. Drug Dev Ind Pharm. 2001;27:373–80.
Curti E, Britto DD, Davis SS, Illum I. Methylation of chitosan with iodomethane: effect of reaction conditions on chemoselectivity and degree of substitution. Macromol Biosci. 2003;3:571–6.
Polnok A, Borchard G, Verhoef JC, Sarisuta N, Junginger HE. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2004;57:77–83.
Loubaki E, Ourevitch M, Sicsic S. Chemical modification of chitosan by glycidyl trimethylammonium chloride: characterization of modified chitosan by 13C- and 1H-NMR spectroscopy. Eur Polym J. 1991;27:311–7.
Daly WH, Manuszak-Guerrini MA. Use of polysaccharide derivatives as anti-infectives substances. Polym Mater Sci Eng. 1998;79:220–1.
Heinze T, Haack V, Rensing S. Starch derivatives of high degree of functionalization: preparation of cationic 2-hydroxypropyltrimethyl ammonium chloride starches. Starch/Staerke. 2004;56:288–96.
Hashem M, Hauser P, Smith B. Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Textile Res J. 2003;73:1017–23.
Sajomsang W, Tantayanon S, Tangpasuthadol V, Daly WH. Quaternization of N-aryl chitosan derivatives: synthesis, characterization, and antibacterial activity. Carbohydrate Research. 2009;344:2502–11.
Lavertu M, Xia Z, Serreqi AN, Berrada M, Rodrigues A, Wang D et al. Validated 1H-NMR method for the determination of the degree of deacetylation of chitosan. J Pharm Biomed Analysis. 2003;32:1149–58.
Kotze AF, Lueben HL, Leeuw BJD, Boer BGD, Verhoef JC, Junginger HE. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J Control Rel. 1998;51:35–46.
Mossman TJ. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Chae Y, Jang MK, Nah JW. Influence of molecular weight on oral absorption of water soluble chitosans. J Control Rel. 2005;102:383–94.
Crini G, Torri G, Guerrini M, Morcellet M, Weltrowski M, Martel B. NMR characterization of N-benzyl sulfonated derivatives of chitosan. Carbohydr Polym. 1997;33:145–51.
Rabea EI, Badawy TME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromol (Review). 2003;4:1457–65.
Daly WH, Manuszak-Guerrini MA. Biocidal chitosan derivatives for cosmetics and pharmaceuticals. US Patent 6,306,835, 23 Oct 2001.
Num CW, Kim YH, Ko SW. Modification of polyacrylonitrile (PAN) fiber by blending with N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride. J Appli Polym Sci. 1999;74:2258–65.
Kim YH, Nam WC, Choi JW, Jang J. Durable antimicrobial treatment of cotton fabrics using n-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride and polycarboxylic acids. J Appli Polym Sci. 2003;88:1567–72.
Lim SH, Hudson SM. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res. 2004;339:313–9.
McEwan G, Jepson M, Hirst B, Simmons N. Polycation induced enhancement of epithelial paracellular permeability is independent of tight junctional characteristics. Biochim Biophys Acta. 1993;1148:51–60.
Thanou MM, Kotze AF, Scharringhausen T, Lueßen HL, Boer DAG, Verhoef JC et al. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J Control Rel. 2000;64:15–25.
Kowapradit J, Opanasopit P, Ngawhiranpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U et al. Methylated N-(4-N, N-dimethylaminobenzyl) chitosan, a novel chitosan derivative, enhances paracellular permeability across intestinal epithelial cells (Caco-2). AAPS Pharm Sci Technol. 2008;9:1143–52.
Acknowledgements
The authors wish to thank Commission of Higher Education (Thailand), The Thailand Research Funds through the Golden Jubilee Ph.D. Program (Grant No. PHD/0114/2550), The National Research Council of Thailand, and Silpakorn University Research and Development Institute for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kowapradit, J., Opanasopit, P., Ngawhirunpat, T. et al. In vitro Permeability Enhancement in Intestinal Epithelial Cells (Caco-2) Monolayer of Water Soluble Quaternary Ammonium Chitosan Derivatives. AAPS PharmSciTech 11, 497–508 (2010). https://doi.org/10.1208/s12249-010-9399-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9399-7