Abstract
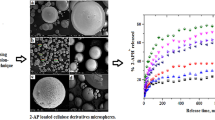
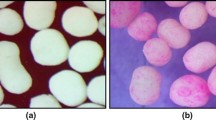
Fluconazole-loaded ethyl cellulose microspheres were prepared by alginate facilitated (water-in-oil)-in-water emulsion technology and the effects of various processing variables on the properties of microspheres were investigated. Scanning electron microscopy revealed spherical nature and smooth surface morphology of the microspheres except those prepared at higher concentration of emulsifiers and higher stirring speeds. The size of microspheres varied between 228 and 592 μm, and as high as 80% drug entrapment efficiency was obtained depending upon the processing variables. When compared up to 2 h, the drug release in pH 1.2 HCl solution was slower than in pH 7.4 phosphate buffer saline solution. However, this trend was reversed at high shear conditions. The microspheres provided extended drug release in alkaline dissolution medium and the drug release was found to be controlled by Fickian-diffusion mechanism. However, the mechanism shifted to anomalous diffusion at high shear rates and emulsifier concentrations. The aging of microspheres did not influence the drug release kinetics. However, the physical interaction between drug and excipients affected the drug dissolution behaviors. X-ray diffractometry (X-RD) and differential scanning calorimetry (DSC) analysis revealed amorphous nature of drug in the microspheres. Fourier transform infrared (FTIR) spectroscopy indicated stable character of fluconazole in the microspheres. The stability testing data also supported the stable nature of fluconazole in the microspheres. The fluconazole extracted from 80% drug-loaded formulation showed good in vitro antifungal activity against Candida albicans. Thus, proper control of the processing variables involved in this modified multiple emulsion technology could allow effective incorporation of slightly water soluble drugs into ethyl cellulose microspheres without affecting drug stability.










Similar content being viewed by others
References
Follonier N, Doelkar E. Biopharmaceutical comparison of oral multiple unit and single unit sustained release dosage forms. STP Pharm Sci. 1992;2:141–58.
Davis SS, Hardy JG, Taylor MJ, Whalley DR, Wilson CG. A comparative study of the gastrointestinal transit of a pellet and tablet formulation. Int J Pharm. 1984;21:167–77.
Hincall AA, Calis S. Microsphere preparation by solvent evaporation method. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. Cambridge, MA: Taylor and Francis; 2000. p. 329–43.
Sanadrap P, Moes AJ. Influence of manufacturing parameters on the size characteristics and release properties of nifedipine from poly (dl-lactide-co-glycolide) microspheres. Int J Pharm. 1993;98:157–64.
Arshady R. Microspheres and microcapsules, a survey of manufacturing techniques. III. Solvent evaporation. Polym Eng Sci. 1990;30:915–24.
Ogawa Y, Yamamoto M, Okada H, Yashiki T, Shimamoto T. A new technique to efficiently entrap leuprolide acetate into microcapsules of poly lactic acid of copolylactic/glycolic acid. Chem Pharm Bull. 1988;36:1095–103.
Parikh RH, Parikh JR, Dubey RR, Soni HN, Kapadia KN. Poly (D, L-lactide-co-glycolide) microspheres containing 5-fluorouracil: optimization of process parameters. AAPS PharmSciTech. 2003;4:14–21.
Sipos P, Csóka I, Srčič S, Pintye-Hódi K, Erõs I. Influence of preparation conditions on the properties of Eudragit microspheres produced by a double emulsion method. Drug Dev Res. 2005;64:41–54.
Alex R, Bodmeier R. Encapsulation of water soluble drugs by a modified solvent evaporation method. I. Effect of process and formulation variables on drug entrapment. J Microencapsul. 1990;3:347–55.
Nagareyan N, Uchida T, Matsuyama K. Preparation and characterization of enteric microspheres containing bovine insulin by a w/o/w emulsion solvent evaporation method. Chem Pharm Bull. 1998;46:1613–7.
Gibaly-El I, Safwat SM, Ahmed MO. Microencapsulation of ketoprofen using w/o/w complex emulsion technique. J Microencapsul. 1996;13:67–87.
J.S. Young, K.I. Chan, and K. Yong-Hee. Preparation method for biodegradable polymeric microspheres using solvent extraction and preparation method for microspheres for treating local inflammation using the same. US Patent no. 6149944 (2000).
Takada S, Yamagata Y, Masaki M, Taira K, Kurokawa T. Sustained release of human growth hormone from ms prepared by a solvent evaporation technique. J Control Rel. 2003;88:229–42.
Yeh MK, Chen JL, Chiang CH. In vivo and in vitro characteristics for insulin-loaded PLA microparticles prepared by w/o/w solvent evaporation method with electrolytes in the continuous phase. J Microencapsul. 2004;21:719–28.
Gibaud S, Gaia A, Astier A. Slow-release melarsoprol microparticles. Int J Pharm. 2002;243:161–6.
Mandal TK, Shekleton M, Onyebueke E, Washington L, Penson T. Effect of formulation and processing factors on the characteristics of biodegradable microcapsules of zidovudine. J Microencapsul. 1996;13:545–57.
Uetrecht J, Walmsley SL. Antimicrobial and antifungal agents that act on cell membrane. In: Kalant H, Roschlau WHE, editors. Principles of medical pharmacology. New York: Oxford University Press; 1998. p. 677.
Parrott EL. Milling. In: Lachman L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. Washington Square, Philadelphia: Lea & Febiger; 1991. p. 28.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Ritger PI, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Rel. 1987;5:37–42.
Jeffery H, Davis SS, O’Hagan DT. The preparation and characterization of poly (lactide-co-glycolide) microparticles. I. Oil-in-water emulsion solvent evaporation. Int J Pharm. 1991;77:169–75.
Benoit MA, Baras B, Gillard J. Preparation and characterization of protein loaded poly (ε-caprolactone) microspheres for oral vaccine delivery. Int J Pharm. 1999;184:73–84.
Rafati H, Coombes AGA, Adler J, Holland J, Davis SS. Protein-loaded poly (dl-lactide-co-glycolide) microparticles for oral administration: formulation, structural and release characteristic. J Control Rel. 1997;43:89–102.
Sah HK, Toddywala R, Chien YW. The influence of biodegradable microcapsule formulations on the controlled release of a protein. J Control Rel. 1994;30:201–11.
Melzer E, Kreuter J, Daniels R. Ethylcellulose: a new type of emulsion stabilizer. Eur J Pharm Biopharm. 2003;56:23–7.
Schügers C, Larucelle N, Wihant N, Grandfils CH. Effect of emulsion stability on the morphology and porosity of semicrystalline poly-1-lactide microparticles prepared by w/o/w double emulsion evaporation. J Control Rel. 1994;32:161–76.
Jiao YY, Ubrich N, Hoffart V, Marchand-Arvier M, Vigneron C, Hoffman M, et al. Preparation and characterization of heparin-loaded polymeric microparticles. Drug Dev Ind Pharm. 2002;28:1033–41.
Schlicher JAM, Postma NS, Zuidema J, Talsma H, Hennik WE. Preparation and characterization of poly (D, L-lactic-co-glycolic acid) microspheres containing desferrixamine. Int J Pharm. 1997;153:235–45.
Jeffery H, Davis SS, O’Hagan DT. Preparation and characterization of poly (lactide-co-glycolide) microparticles: II: entrapment of a model protein using a (water in oil) in water emulsion solvent evaporation technique. Pharm Res. 1993;10:362–8.
Leo E, Pecquet S, Rojas J, Couvruer P, Fattal E. Changing the pH of the external aqueous phase may modulate protein entrapment and delivery from poly (lactide-co-glycolide) microspheres prepared by w/o/w solvent evaporation method. J Microencapsul. 1998;15:421–30.
Yeh MK, Tung SM, Lu DW, Chiang CH. Formulation factors for preparing ocular biodegradable delivery system of 5-fluorouracil microparticles. J Microencapsul. 2001;18:507–19.
Crotts G, Gwan Park T. Protein delivery from poly (lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul. 1998;15:699–713.
Blanco-Prieto MJ, Delie F, Fattal E, Tartar A, Puisieux F, Gulik A, et al. Characterization of V3 BRU peptide-loaded small PLGA microspheres prepared by a (w1/o/w2) emulsion solvent evaporation method. Int J Pharm. 1994;111:137–45.
Dash AK, Elmquist WF. Fluconazole. In: Brittain H, editor. Analytical profiles of drug substances and excipients. San Diego: Academic; 2001. p. 67–113.
Pignatello R, Spadaro D, Vandelli MA, Forni F, Puglisi G. Characterization of the mechanism of interaction in ibuprofen–eudragit RL100® coevaporates. Drug Dev Ind Pharm. 2004;30:277–88.
Kim K-H, Kim K-S, Lim J-S, Shin J-S, Chung K-H. Effect of dissolution properties of p-aminosalicylic acid with chitin and chitosan mixtures. Polymer (Korea). 1988;12:56–62.
Carrillo-Munoz AJ, Quindos G, Tur C, Ruesga MT, Miranda Y, delValle O, et al. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J Antimicrob Chemother. 1999;44:397–401.
Acknowledgments
The authors are grateful to the authority of Gupta College of Technological Sciences, Asansol, India and Jadavpur University, Department of Pharmaceutical Technology, Kolkata, India for providing necessary facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maiti, S., Dey, P., Kaity, S. et al. Investigation on Processing Variables for the Preparation of Fluconazole-Loaded Ethyl Cellulose Microspheres by Modified Multiple Emulsion Technique. AAPS PharmSciTech 10, 703–715 (2009). https://doi.org/10.1208/s12249-009-9257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9257-7