Abstract
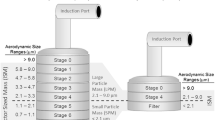
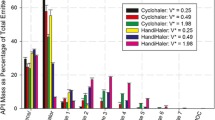
The abbreviated impactor measurement (AIM) concept is a potential solution to the labor-intensive full-resolution cascade impactor (CI) methodology for inhaler aerosol aerodynamic particle size measurement. In this validation study, the effect of increasing the internal dead volume on determined mass fractions relating to aerodynamic particle size was explored with two abbreviated impactors both based on the Andersen nonviable cascade impactor (ACI) operating principle (Copley fast screening Andersen impactor [C-FSA] and Trudell fast screening Andersen impactor [T-FSA]). A pressurized metered dose inhaler-delivered aerosol producing liquid ethanol droplets after propellant evaporation was chosen to characterize these systems. Measures of extrafine, fine, and coarse particle mass fractions from the abbreviated systems were compared with corresponding data obtained by a full-resolution ACI. The use of liquid ethanol-sensitive filter paper provided insight by rendering locations visible where partly evaporated droplets were still present when the “droplet-producing” aerosol was sampled. Extrafine particle fractions based on impactor-sized mass were near equivalent in the range 48.6% to 54%, comparing either abbreviated system with the benchmark ACI-measured data. The fine particle fraction of the impactor-sized mass determined by the T-FSA (94.4 ± 1.7%) was greater than using the C-FSA (90.5 ± 1.4%) and almost identical with the ACI-measured value (95.3 ± 0.4%). The improved agreement between T-FSA and ACI is likely the result of increasing the dead space between the entry to the induction port and the uppermost impaction stage, compared with that for the C-FSA. This dead space is needed to provide comparable conditions for ethanol evaporation in the uppermost parts of these impactors.



Similar content being viewed by others
Abbreviations
- ACI:
-
Andersen cascade impactor
- AIM:
-
abbreviated impactor measurement
- API:
-
active pharmaceutical ingredient
- APSD:
-
aerodynamic particle size distribution
- BDP:
-
beclomethasone dipropionate
- CI:
-
cascade impactor
- C-FSA:
-
Copley fast screening Andersen impactor
- CPF*:
-
coarse particle fraction (based on impactor-sized mass)
- EPF*:
-
extrafine particle fraction (based on impactor-sized mass)
- FPF*:
-
fine particle fraction (based on impactor-sized mass)
- MMAD:
-
mass median aerodynamic diameter of impactor-sized aerosol
- pMDI:
-
pressurized metered dose inhaler
- T-FSA:
-
Trudell fast screening Andersen impactor
References
J. P. Mitchell, M. W. Nagel, V. Avvakoumova, H. Mackay, and R. Ali. The abbreviated impactor measurement (AIM) concept: part-1—influence of particle bounce and re-entrainment—evaluation with a mid-size range “dry” pressurized metered dose inhaler-based formulation. AAPS PharmSciTech. in press (2008).
European Pharmacopeia—section 2.9.18—preparations for inhalation: aerodynamic assessment of fine particles. European Pharmacopeia: 5th Edn. Council of Europe, 67075 Strasbourg, France, pp. 2799–2811 (2005).
United States Pharmacopeia; USP 30-NF 25; Chapter 601—physical tests and determinations: aerosols. United States Pharmacopeia, Rockville, MD, USA, pp. 220–240 (2007).
J. P. Mitchell. The abbreviated impactor measurement (AIM) concept for aerodynamic particle size distribution (APSD) in a quality-by-design (QbD) environment. Proc. Biennial IPAC-RS Conference, Bethesda, MD, USA. 2008. Available at http://www.ipacrs.com/ipac2008.html. Accessed 5 October 2008.
S. W. Stein, and J. S. Stefely. Reinventing metered dose inhalers: from poorly efficient CFC MDIs to highly efficient HFA MDIs. Drug Deliv. Technol. 31:46–51 (2003).
S. W. Stein, and P. B. Myrdal. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J. Pharm. Sci. 93:2158–2175 (2004).
P. B. Myrdal, E. Mogallian, J. P. Mitchell, M. Nagel, C. Wright, B. Kiser, M. Prell, M. Woessner, and S. W. Stein. Application of heated inlet extensions to the TSI 3306/3321 system: comparison with the Andersen cascade impactor and next generation impactor. J. Aerosol Med. 194:543–554 (2006).
P. B. Myrdal, S. W. Stein, E. Mogalian, W. Hoye, and A. Gupta. Comparison of the TSI model 3306 impactor inlet with the Andersen cascade impactor: solution metered dose inhalers. Drug Dev. Ind. Pharm. 30:859–868 (2004).
C. Leach. Enhanced drug delivery through reformulating MDIs with HFA propellants—drug deposition and its effect on preclinical and clinical programs. In R. N. Dalby, P. R. Byron, and S. J. Farr (eds.), Respiratory Drug Delivery—V, Interpharm, Buffalo Grove, IL, 1996, pp. 133–144.
S. W. Stein. Aiming for a moving target: challenges with impactor measurements of MDI aerosols. Int. J. Pharm. 3551–2:53–61 (2007).
US Federal Drug Administration (FDA). Draft guidance: metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products chemistry, manufacturing and controls documentation. Docket 98D-0997 (1998).
M. N. Nasr, D. L. Ross, and N. C. Miller. Effect of drug load and plate coating on the particle size distribution of a commercial albuterol metered dose inhaler (MDI) determined using the Andersen and Marple–Miller cascade impactors. Pharm. Res. 1410:1437–1443 (1997).
A. Gupta, S. W. Stein, and P. B. Myrdal. Balancing ethanol cosolvent concentration with product performance in 134a-based pressurized metered dose inhalers. J. Aerosol Med. 162:167–174 (2003).
K. W. Stapleton, and W. H. Finlay. Undersizing of droplets from a vented nebuliser caused by aerosol heating during transit through an Andersen impactor. J. Aerosol Sci. 301:105–109 (1999).
M. Copley, M. Smurthwaite, D. L. Roberts, and J. P. Mitchell. Revised internal volumes to those provided by Mitchell JP and Nagel MW in “Cascade Impactors for the Size Characterization of Aerosols From Medical Inhalers: Their Uses and Limitations”. J. Aerosol Med. 183:364–366 (2005).
V. Chavan, and R. Dalby. Novel system to investigate the effects of inhaled volume and rates of rise in simulated inspiratory air flow on fine particle output from a dry powder inhaler. AAPS PharmSci. 42:E6 (2002)Available at http://www.aapspharmsci.org/.
Acknowledgements
The authors acknowledge the support of Copley Scientific Ltd. for the C-FSA and the advice and support of Mark Copley and Daryl Roberts (MSP Corp., St. Paul, MN, USA) during the development and execution of this investigation. They also wish to thank Steven Stein (3M Drug Delivery Systems, St. Paul, MN, USA) for the supply of ethanol-sensitive paper as well as for additional discussions as the work progressed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitchell, J.P., Nagel, M.W., Avvakoumova, V. et al. The Abbreviated Impactor Measurement (AIM) Concept: Part II—Influence of Evaporation of a Volatile Component—Evaluation with a “Droplet-Producing” Pressurized Metered Dose Inhaler (pMDI)-Based Formulation Containing Ethanol as Cosolvent. AAPS PharmSciTech 10, 252–257 (2009). https://doi.org/10.1208/s12249-009-9201-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9201-x