Abstract
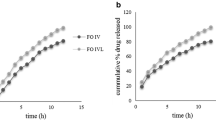
A biphasic gastroretentive floating drug delivery system with multiple-unit mini-tablets based on gas formation technique was developed to maintain constant plasma level of a drug concentration within the therapeutic window. The system consists of loading dose as uncoated core units, and prolonged-release core units are prepared by direct compression process; the latter were coated with three successive layers, one of which is seal coat, an effervescent (sodium bicarbonate) layer, and an outer polymeric layer of polymethacrylates. The formulations were evaluated for quality control tests, and all the parameters evaluated were within the acceptable limits. The system using Eudragit RL30D and combination of them as polymeric layer could float within acceptable time. The drug release was linear with the square root of time. The rapid floating and the controlled release properties were achieved in this present study. When compared with the theoretical release profile, the similarity factor of formulation with coating of RS:RL (1:3)–7.5%, was observed to be 74, which is well fitted into zero-order kinetics confirming that the release from formulation is close to desired release profile. The stability samples showed no significant change in dissolution profiles (p > 0.05). In vivo gastric residence time was examined by radiograms, and it was observed that the units remained in the stomach for about 5 h.






Similar content being viewed by others
References
A. C. Shah. Design of oral sustained release drug delivery systems: in vitro/in vivo Considerations. In A. Yacobi, and E. Halperin-Walega (eds.), Oral Sustained Release Formulation Design and Evaluation, Pergamon, New York, 1988, pp. 35–56.
A. Rubinstein, V. H. K. Li, P. Gruber, and J. R. Robinson. Gastrointestinal-physiological variables affecting the performance of oral sustained release dosage forms. In A. Yacobi, and E. Halperin-Walega (eds.), Oral Sustained Release Formulation Design and Evaluation, Pergamon, New York, 1988, pp. 125–156.
M. Mayersohn. Principles of drug absorption. In G. C. Banker, and C. T. Rhodes (eds.), Modern Pharmaceutics, Marcel Dekker, New York, 1979, pp. 23–89.
P. K. Gupta, and J. R. Robinson. Oral controlled-release delivery. In A. Kydonieus (ed.), Treatise on Controlled Drug Delivery, Marcel Dekker, New York, 1992, pp. 255–313.
N. Rouge, E. T. Cole, E. Doelker, and P. Buri. Screening of potentially floating excipients for Minitablets. S.T.P. Pharm. Sci. 7:386–392 (1997).
L. Maggi, E. O. Machiste, M. L. Torre, and U. Conte. Formulation of biphasic release tablets containing slightly soluble drugs. Eur. J. Pharm. Biopharm. 48:37–42 (1999).
S. Somade, and K. Singh. Comparative evaluation of wet granulation and direct compression methods for preparation of controlled release ranitidine HCL tablets. Indian J. Pharm. Sci. 64:285 (2002).
K. Lauritsen. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases. Clin. Pharmacokinet. 1911–31:94–125 (1990).
S. Grant. Ranitidine: an updated review of its pharmacodynamics and pharmacokinetic properties and therapeutic use in peptic ulcer and other allied diseases. Drugs. 37:801–870 (1989).
A. Basit, and L. Lacey. Colonic metabolism of ranitidine: implications for its delivery and absorption. Int. J. Pharm. 227:157–165 (2001).
M. Coffin, and A. Parr, inventors. Glaxo Inc. Ranitidine solid dosage form. US patent 5 407 687. April 18, 1995.
J. G. Wagner. Biopharmaceutics and pharmacokinetics. 1st ed. Org. Intelligence publications, 22, 1971, pp. 148–157.
A. M. Hamid, M. S. Harris, T. Jaweria, and I. Y. Rabia. Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: a technical note. AAPS PharmSciTech. 7:(3) Article 78 (2006).
P. Costa, and J. M. S. Lobo. Modeling and comparison dissolution profiles. Int. J. Pharm. 13:123–133 (2001).
J. W. Moore, and H. H. Flanner. Mathematical comparison of curves with an emphasis on in- vitro dissolution profiles. Pharm. Tech. 20:64–74 (1996).
P. Colombo, R. Bettini, P. Santi, and N. A. Peppas. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. PSTT. 3:198–204 (2000).
S. Kiil, and K. Dam-Johansen. Controlled drug delivery from swellable Hydroxyl propyl methylcellulose matrices: model-based analysis of observed radial front movements. J. Contr. Release. 90:1–21 (2003).
I. Krögel, and R. Bodmeier. Floating or pulsatile drug delivery systems based on coated effervescent cores. Int. J. Pharm. 187:175–184 (1999).
V. Iannuccelli, G. Coppi, M. T. Bernabei, and R. Cameroni. Air compartment multipleunit system for prolonged gastric residence. Part I. Formulation study. Int. J. Pharm. 174:47–54 (1998).
I. Ghebre-Sellassie, R. U. Nesbitt, and J. Wang. Eudragit aqueous dispersions as pharmaceutical controlled release coatings. In J. W. McGinity (ed.), Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms, 2nd ed., Marcel Dekker, New York, pp. 267–286.
K. H. Bauer, K. Lehmann, H. P. Osterwald, and G. Rothgang. Coated Pharmaceutical Dosage Forms, Medpharm Scientific, Stuttgart, 1998, pp. 63–119.
M. Ichigawa, S. Watanabe, and Y. Miyake. A new multiple-unit oral floating dosage system. I. Preparation and in vitro evaluation of floating and sustained release characteristics. J. Pharm. Sci. 80:1062–1066 (1991).
Acknowledgments
The authors would like to thank Dr Reddy’s Laboratories (Hyderabad, India) for providing the gift sample of ranitidine hydrochloride, and one of the authors (Meka Lingam) is thankful to the UGC India for providing Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lingam, M., Ashok, T., Venkateswarlu, V. et al. Design and Evaluation of a Novel Matrix Type Multiple Units as Biphasic Gastroretentive Drug Delivery Systems. AAPS PharmSciTech 9, 1253–1261 (2008). https://doi.org/10.1208/s12249-008-9173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9173-2