Abstract
Clinical trials are the gatekeepers and bottlenecks of progress in medicine. In recent years, they have become increasingly complex and expensive, driven by a growing number of stakeholders requiring more endpoints, more diverse patient populations, and a stringent regulatory environment. Trial designers have historically relied on investigator expertise and legacy norms established within sponsor companies to improve operational efficiency while achieving study goals. As such, data-driven forecasts of operational metrics can be a useful resource for trial design and planning. We develop a machine learning model to predict clinical trial operational efficiency using a novel dataset from Roche containing over 2,000 clinical trials across 20 years and multiple disease areas. The data includes important operational metrics related to patient recruitment and trial duration, as well as a variety of trial features such as the number of procedures, eligibility criteria, and endpoints. Our results demonstrate that operational efficiency can be predicted robustly using trial features, which can provide useful insights to trial designers on the potential impact of their decisions on patient recruitment success and trial duration.
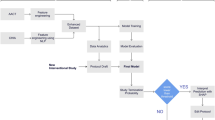
Graphical Abstract




Similar content being viewed by others
Code Availability
The trained machine learning model is available at https://github.com/kevinwu23/clinical-trial-efficiency.
REFERENCES
Kelly D, Spreafico A, Siu LL. Increasing operational and scientific efficiency in clinical trials. Br J Cancer. 2020;123(8):1207–8.
Rosenblatt M. The large pharmaceutical company perspective. N Engl J Med. 2017;376(1):52–60.
Martin L, Hutchens M, Hawkins C, Radnov A. How much do clinical trials cost. Nat Rev Drug Discov. 2017;16(6):381–2.
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med. 2016;176(12):1826–33.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–86.
Getz K. Improving protocol design feasibility to drive drug development economics and performance. Int J Environ Res Public Health. 2014;11(5):5069–80.
Kaitin KI. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther. 2010;87(3):356–61.
Ledford H. Translational research: 4 ways to fix the clinical trial. Nat News. 2011;477(7366):526–8.
Liu R, Rizzo S, Whipple S, Pal N, Pineda AL, Lu M, et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592(7855):629–33.
Shenoy P. Multi-regional clinical trials and global drug development. Perspect Clin Res. 2016;7(2):62.
Song SY, Chee D, Kim E. Strategic inclusion of regions in multiregional clinical trials. Clin Trials. 2019;16(1):98–105.
Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun. 2018;11:156–64.
Allison M. Reinventing clinical trials. Nat Biotechnol. 2012 Jan 9;30(1):41–9.
Cavallo C, Labib MA, Honea N, Nakaji P. Enrollment-to-screening ratio: an undervalued data in randomized clinical trials. Neurosurgery. 2018.
Craven BC, Balioussis C, Hitzig SL, Moore C, Verrier MC, Giangregorio LM, et al. Use of screening to recruitment ratios as a tool for planning and implementing spinal cord injury rehabilitation research. Spinal Cord. 2014;52(10):764–8.
Blanton S, Morris DM, Prettyman MG, McCulloch K, Redmond S, Light KE, et al. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther. 2006;86(11):1520–33.
Harris-Brown TM, Paterson DL. Reporting of pre-enrolment screening with randomized clinical trials: a small item that could impact a big difference. Perspect Clin Res. 2015;6(3):139.
Giffin RB, Lebovitz Y, English RA. Transforming clinical research in the United States: challenges and opportunities: workshop summary: National Academies Press; 2010.
Lamberti MJ, Smith Z, Henry R, Howe D, Goodwin M, Williams A, et al. Benchmarking patient recruitment and retention practices. Ther Innov Regul Sci. 2021;55(1):19–32.
Stergiopoulos S, Calvert SB, Brown CA, Awatin J, Tenaerts P, Holland TL, et al. Cost drivers of a hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia phase 3 clinical trial. Clin Infect Dis. 2018;66(1):72–80.
McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(1):1–8.
Keith SJ. Evaluating characteristics of patient selection and dropout rates. J Clin Psychiatry. 2001;62:11–6.
Dicesare J. Improve the clinical trial startup process with just-in-time site activation. Applied Clinical Trials [Internet]. 2014 Aug 19; Available from: https://www.appliedclinicaltrialsonline.com/view/improve-clinical-trial-startup-process-just-time-site-activation
Getz KA. Characterizing the real cost of site regulatory compliance. Appl Clin Trials. 2015;24(6/7):18.
Eichler H-G, Sweeney F. The evolution of clinical trials: can we address the challenges of the future? Clin Trials. 2018;15(1_suppl):27–32.
Smuck B, Bettello P, Berghout K, Hanna T, Kowaleski B, Phippard L, et al. Ontario protocol assessment level: clinical trial complexity rating tool for workload planning in oncology clinical trials. J Oncol Pract. 2011;7(2):80–4.
Cunanan KM, Gonen M, Shen R, Hyman DM, Riely GJ, Begg CB, et al. Basket trials in oncology: a trade-off between complexity and efficiency. J Clin Oncol. 2017;35(3):271.
Malik L, Lu D. Increasing complexity in oncology phase I clinical trials. Invest New Drugs. 2019;37(3):519–23.
Yuan G, Wang L, Li J, Feng H, Ji J, Gu W, et al. Complexity in clinical trials: blind spots, misleading criteria, winners and losers. Clin Cancer Drugs. 2020;7(1):3–15.
Thadani SR, Weng C, Bigger JT, Ennever JF, Wajngurt D. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc. 2009;16(6):869–73.
Frank G. Current challenges in clinical trial patient recruitment and enrollment. SoCRA Source. 2004;2(February):30–8.
Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS. Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. 2016;7(3):137.
Dilts DM, Sandler AB, Baker M, Cheng SK, George SL, Karas KS, et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: the Case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24(28):4553–7.
Getz KA, Wenger J, Campo RA, Seguine ES, Kaitin KI. Assessing the impact of protocol design changes on clinical trial performance. Am J Ther. 2008;15(5):450–7.
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–56.
Boericke K, Gwinn B. Planned to perfection. Int Clin Trials. 2010;17(8):26–30.
Andersen JW, Fass R, van der Horst C. Factors associated with early study discontinuation in AACTG studies, DACS 200. Contemp Clin Trials. 2007;28(5):583–92.
Tseo Y, Salkola MI, Mohamed A, Kumar A, Abnousi F. Information extraction of clinical trial eligibility criteria. ArXiv Prepr ArXiv200607296. 2020;
Liu H, Chi Y, Butler A, Sun Y, Weng C. A knowledge base of clinical trial eligibility criteria. J Biomed Inform. 2021;117:103771.
Munos B, Niederreiter J, Riccaboni M. Improving the prediction of clinical success using machine learning. 2020;
Feijoo F, Palopoli M, Bernstein J, Siddiqui S, Albright TE. Key indicators of phase transition for clinical trials through machine learning. Drug Discov Today. 2020;25(2):414–21.
Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, et al. Lightgbm: A highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. 2017;30:3146–54.
Feurer M, Klein A, Eggensperger K, Springenberg JT, Blum M, Hutter F. Auto-sklearn: efficient and robust automated machine learning. In: Automated Machine Learning. Springer, Cham; 2019. p. 113–34.
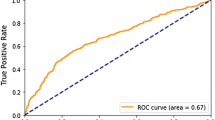
Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247(18):2543–6.
Apley DW, Zhu J. Visualizing the effects of predictor variables in black box supervised learning models. J R Stat Soc Ser B Stat Methodol. 2020;82(4):1059–86.
Acknowledgements
We thank Ernesto Guarin, Nicole Kim, Katelyn Bechler, James Harper, Jacek Basta, and Jennifer Copping for their helpful feedback.
Funding
This work was funded by Genentech, part of the Roche Group.
Author information
Authors and Affiliations
Contributions
KW and EW were involved in the design, analysis, and interpretation of data. JZ was involved in the design and interpretation of the data. MD, NC, ML, MD, and KK were involved in feedback on content and interpretation. HR, RL, MG, NP, CH, SR, and RC were involved in the review and feedback on content.
Corresponding author
Ethics declarations
Competing Interest
MDA, NC, ML, MD, KK, HR, MG, NP, CH, SR, and RC are employees of Roche.
Additional information
Guest Editors: Lawrence Yu, Hao Zhu and Qi Liu
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, K., Wu, E., DAndrea, M. et al. Machine Learning Prediction of Clinical Trial Operational Efficiency. AAPS J 24, 57 (2022). https://doi.org/10.1208/s12248-022-00703-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00703-3