Abstract
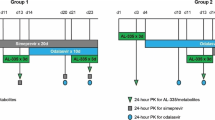
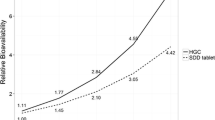
The aim of this study was to characterize the pharmacokinetic drug–drug interaction (DDI) between simeprevir (NS3/4A protease inhibitor) and odalasvir (NS5A inhibitor) after oral administration to support the design and dose selection of clinical studies with this combination for the treatment of chronic hepatitis C infection (HCV). Simeprevir and odalasvir plasma concentrations from 30 healthy subjects receiving these drugs in monotherapy as well as in combination were pooled and analyzed using a population pharmacokinetic modeling approach. Previous pharmacokinetic models developed to characterize the pharmacokinetics for each drug were used as starting point. The dual effect of simeprevir and odalasvir on their pharmacokinetic parameters was explored. Simulations were performed to assess the impact of the DDI on exposure parameters. In presence of odalasvir, the relative bioavailability of simeprevir increased by 26% and the apparent clearance was reduced following competitive inhibition depending on odalasvir plasma concentrations, with an inhibitory constant (Ki) estimated to be 1610 ng/mL. The apparent odalasvir clearance was reduced by simeprevir plasma concentrations following an Imax model, characterized by a maximum inhibitory effect of 46.7% and an IC50 of 257 ng/mL. Model-based simulations indicated that both Cmax and AUC24h increased for both drugs, when co-administered. The pharmacokinetic model adequately describes the time course of plasma concentrations and their variability when simeprevir and/or odalasvir were orally administered. This model can be used as a first step to predict the exposures of concomitant administration of simeprevir and odalasvir in HCV-infected subjects. Data from study AL355-602 (NCT02512562) were used for this analysis.





Similar content being viewed by others
References
Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22(10):833–8. https://doi.org/10.1016/j.cmi.2016.07.035.
Pawlotsky J-M, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132(5):1979–98. https://doi.org/10.1053/j.gastro.2007.03.116.
EASL Recommendations on Treatment of Hepatitis C. European Association for the Study of the Liver. J Hepatol. 2018. https://doi.org/10.1016/j.jhep.2018.03.026.
Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalshy VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384(9941):403–113. https://doi.org/10.1016/S0140-6736(14)60494-3.
Ouwerkerk-Mahadevan S, Simion A, Mortier S, Peeters M, Beumont-Mauviel M. The effect of food and different meal types on the bioavailability of simeprevir (TMC435), an HCV protease inhibitor in clinical development. 8th international workshop on clinical pharmacology of hepatitis therapy. Cambridge, MA, USA, June 26–27, 2013.
Ouwerkerk-Mahadevan S, Snoeys J, Peeters M, Beumont-Mauviel M, Simion A. Drug–drug interactions with the NS3/4A protease inhibitor simeprevir. Clin Pharmacokinet. 2016;55(2):197–208. https://doi.org/10.1007/s40262-015-0314-y.
OLYSIO (simeprevir) capsules: prescribing information. Titusville: Janssen Pharmaceuticals, Inc; 2017. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/OLYSIO-pi.pdf. Published Nov 2017. Accessed 27 Jun 2018.
Reesink HW, Fanning GC, Farha KA, Weegink C, Van Vliet A, Van’t Klooster G, et al. Rapid HCV-RNA decline with once daily TMC435: a phase I study in healthy volunteers and hepatitis C patients. Gastroenterology. 2010;138(3):913–21. https://doi.org/10.1053/j.gastro.2009.10.033.
Viberg A, Petersson K, Hoeben E, Brochot A. A population PK model for simperevir in healthy volunteers and patients. 24th population approach Group in Europe meeting, June 2015; Crete, Greece http://www.page-meeting.org/?abstract=3368.
Yang G, Wiles J, Patel D, Zhao Y, Fabrycki J, Weinheimer S, et al. Preclinical characteristics of ACH-3102; a novel NS5A inhibitor with improved potency against genotype-1A virus and variants resistant to 1st generation NS5A inhibitors. J Hepatol. 2012;56(2):S330. https://doi.org/10.1016/S0168-8278(12)60857-5.
Gane E, Schwabe C, Mader M, Suri V, Donohue M, Huang M, et al. Sustained virologic response after ACH-3102 and sofosbuvir treatment for 8 or 6 weeks: a phase 2 “proxy” study. European Association for the Study of the Liver, The International Liver Congress™. Vienna, Austria, April 22–26, 2015. Abstract P017.
Ackaert O, Valade E, Ouwerkerk-Mahadevan S, Kakuda T, Perez-Ruixo J, Hoeben E. Dose-selection of odalasvir for use in combination with AL-335 and simeprevir in a phase 2A study using a population pharmacokinetic modelling and simulation approach. 18th international workshop on clinical pharmacology of antiviral therapy, June 2017; Chicago, USA. http://www.natap.org/2017/Pharm/Pharm_44.htm. Accessed 18 Dec 2017.
Kakuda TN, McClure MW, Westland C, Vuong J, Homery M-C, Poizat G, et al. Pharmacokinetics, safety and tolerability of the 2- and 3-direct-acting antiviral combination of AL-335, odalasvir and simeprevir in healthy subjects. Pharmacol Res Perspect. 2018;6(3):e00395. https://doi.org/10.1002/prp2.395.
Lehr T, Staab A, Trommeshauser D, Schaefer HG, Kloft C. Semi-mechanistic population pharmacokinetic drug–drug interaction modelling of a long half-life substrate and itraconazole. Clin Pharmacokinet. 2010;49(1):53–66. https://doi.org/10.2165/11317210-000000000-00000.
Beal S, Boeckman A, Scheiner L. NONMEM user’s guides. San Francisco: University of California at San Francisco; 1988.
R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. ISBN 3-900051-07-0, URL http://www.R-project.org
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Prog Biomed. 2004;75(2):85–94. https://doi.org/10.1016/j.cmpb.2003.11.003.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26. https://doi.org/10.1007/s10928-007-9066-0.
Wilkins JJ, Savic RM, Karlsson MO, Langdon G, McIlleron H, Pillai G, et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob Agents Chemother. 2008;52(6):2138–48. https://doi.org/10.1128/AAC.00461-07.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–23.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):87–109. https://doi.org/10.1002/psp4.12161.
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28:171–92.
Thakkar N, Slizgi JR, Brouwer KLR. Effect of liver disease on hepatic transporter expression and function. J Pharm Sci. 2017;106(9):2282–94. https://doi.org/10.1016/j.xphs.2017.04.053.
Gane E, Stedman C, Schwabe C, et al. Short duration AL-335, odalasvir, with/without simeprevir, in patients with HCV GT1 or 3 infection without cirrhosis. Hepatology. 2018. https://doi.org/10.1002/hep.30126.
Acknowledgements
The authors thank Eef Hoeben for her comments and support during the analysis. In addition, the authors would like to thank the healthy volunteers, investigators, and their medical, nursing, and laboratory staff who participated in the clinical trials included in the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure Section
M.W.M. was an employee of Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies at the time this study was conducted.
Rights and permissions
About this article
Cite this article
Valade, E., Valenzuela, B., Kakuda, T.N. et al. Characterizing the Pharmacokinetic Interaction Between Simeprevir and Odalasvir in Healthy Volunteers Using a Population Modeling Approach. AAPS J 20, 111 (2018). https://doi.org/10.1208/s12248-018-0271-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-018-0271-0