Abstract
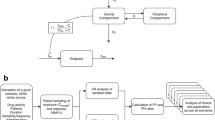
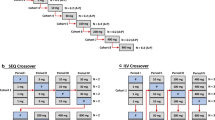
The exposure-response relationship of combinatory drug effects can be quantitatively described using pharmacodynamic interaction models, which can be used for the selection of optimal dose combinations. The aim of this simulation study was to evaluate the reliability of parameter estimates and the probability for accurate dose identification for various underlying exposure-response profiles, under a number of different phase II designs. An efficacy variable driven by the combined exposure of two theoretical compounds was simulated and model parameters were estimated using two different models, one estimating all parameters and one assuming that adequate previous knowledge for one drug is readily available. Estimation of all pharmacodynamic parameters under a realistic, in terms of sample size and study design, phase II trial, proved to be challenging. Inaccurate estimates were found in all exposure-response scenarios, except for situations where no pharmacodynamic interaction was present, with the drug potency and interaction parameters being the hardest to estimate. When previous knowledge of the exposure-response relationship of one of the monocomponents is available, such information should be utilized, as it enabled relevant improvements in parameter estimation and in correct dose identification. No general trends for classification of the performance of the tested study designs across different scenarios could be identified. This study shows that pharmacodynamic interactions models can be used for the exposure-response analysis of clinical endpoints especially when accompanied by appropriate dose selection in regard to the expected drug potencies and appropriate trial size and if information regarding the exposure-response profile of one monocomponent is available.








Similar content being viewed by others
References
Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1–2):34–42.
Bliss CI. The toxicity of poisons applied Jointly1. Ann Appl Biol. 1939;26(3):585–615.
Loewe SH. Effect of combinations: mathematical basis of problem. Arch Exp Pathol Pharmacol. 1926(114):313–26.
Geary N. Understanding synergy. Am J Physiol Endocrinol Metab. 2013;304(3):E237–53.
Lee JY, Garnett CE, Gobburu JV, Bhattaram VA, Brar S, Earp JC, et al. Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin Pharmacokinet. 2011;50(10):627–35.
Overgaard RV, Ingwersen SH, Tornøe CW. Establishing good practices for exposure-response analysis of clinical endpoints in drug development. CPT Pharmacometrics Syst Pharmacol. 2015;4(10):565–75.
Powell JR, Gobburu JV. Pharmacometrics at FDA: evolution and impact on decisions. Clin Pharmacol Ther. 2007;82(1):97–102.
Koch G, Schropp J, Jusko WJ. Assessment of non-linear combination effect terms for drug-drug interactions. J Pharmacokinet Pharmacodyn. 2016;43(5):461–79.
Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47(2):331–85.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81.
Papathanasiou T, Juul RV, Heegaard AM, Kreilgaard M, Lund TM. Co-administration of morphine and gabapentin leads to dose dependent synergistic effects in a rat model of postoperative pain. Eur J Pharm Sci. 2016;82:97–105.
Herrick TM, Million RP. Tapping the potential of fixed-dose combinations. Nat Rev Drug Discov. 2007;6(7):513–4.
Kong M, Lee JJ. A generalized response surface model with varying relative potency for assessing drug interaction. Biometrics. 2006;62(4):986–95.
Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL. Response surface model for anesthetic drug interactions. Anesthesiology. 2000;92(6):1603–16.
Twarog NR, Stewart E, Hammill CV, Shelat AA. BRAID: a unifying paradigm for the analysis of combined drug action. Sci Rep. 2016;6:25523.
Papathanasiou T, Juul RV, Gabel-Jensen C, Kreilgaard M, Heegaard AM, Lund TM. Quantification of the pharmacodynamic interaction of morphine and gabapentin using a response surface approach. AAPS J. 2017;
Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. N Engl J Med. 2011;364(11):985–7.
Zhu H, Wang Y. Evaluation of false positive rate based on exposure-response analyses for two compounds in fixed-dose combination products. J Pharmacokinet Pharmacodyn. 2011;38(6):671–96.
Girgis S, Pai SM, Girgis IG, Batra VK. Pharmacodynamic parameter estimation: population size versus number of samples. AAPS J. 2005;7(2):46.
Team RC. R: a language and environment for statistical computing. 2015.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2009.
Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40(1):1–29.
Pai SM, Girgis S, Batra VK, Girgis IG. Population pharmacodynamic parameter estimation from sparse sampling: effect of sigmoidicity on parameter estimates. AAPS J. 2009;11(3):535–40.
Musuamba FT, Manolis E, Holford N, Cheung S, Friberg LE, Ogungbenro K, et al. Advanced methods for dose and regimen finding during drug development: summary of the EMA/EFPIA workshop on dose finding (London 4-5 December 2014). CPT Pharmacometrics Syst Pharmacol. 2017;6(7):418–29.
Center for Drug Evaluation and Research F. Guidance for industry, exposure-response relationships—study design, data analysis, and regulatory applications. 2003.
Bornkamp B, Bretz F, Dmitrienko A, Enas G, Gaydos B, Hsu CH, et al. Innovative approaches for designing and analyzing adaptive dose-ranging trials. J Biopharm Stat. 2007;17(6):965–95.
Dutta S, Matsumoto Y, Ebling WF. Is it possible to estimate the parameters of the sigmoid Emax model with truncated data typical of clinical studies? J Pharm Sci. 1996;85(2):232–9.
U.S. Department of Health and Human Services, Food and Drug Administration. Code of Federal Regulations Title 21, Sect. 300.50. Fixed-combination prescription drugs for humans. 2014.
Aoki Y, Röshammar D, Hamrén B, Hooker AC. Model selection and averaging of nonlinear mixed-effect models for robust phase III dose selection. J Pharmacokinet Pharmacodyn. 2017;44(6):581–97.
Miller F, Guilbaud O, Dette H. Optimal designs for estimating the interesting part of a dose-effect curve. J Biopharm Stat. 2007;17(6):1097–115.
Kågedal M, Karlsson MO, Hooker AC. Improved precision of exposure-response relationships by optimal dose-selection. Examples from studies of receptor occupancy using PET and dose finding for neuropathic pain treatment. J Pharmacokinet Pharmacodyn. 2015;42(3):211–24.
Strömberg EA, Hooker AC. The effect of using a robust optimality criterion in model based adaptive optimization. J Pharmacokinet Pharmacodyn. 2017;44(4):317–24.
Ryeznik Y, Sverdlov O, Hooker AC. Adaptive optimal designs for dose-finding studies with time-to-event outcomes. AAPS J. 2017;20(1):24.
Acknowledgments
The authors would like to thank the anonymous reviewers for their constructive criticism and feedback for improving this work. R.V.O and A.S. are full-time employees of Novo Nordisk A/S.
Funding
This work was supported by the Novo Nordisk STAR Fellowship Programme and the Innovation Fund Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Papathanasiou, T., Strathe, A., Hooker, A.C. et al. Feasibility of Exposure-Response Analyses for Clinical Dose-Ranging Studies of Drug Combinations. AAPS J 20, 64 (2018). https://doi.org/10.1208/s12248-018-0226-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-018-0226-5