Abstract
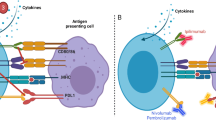
The development of novel therapies that can harnass the immune system to eradicate cancer is an area of intensive research. Several new biopharmaceuticals that target the immune system rather than the tumor itself have recently been approved and fundamentally transformed treatment of many cancer diseases. This success has intensified the search for new targets and modalities that could be developed as even more effective therapeutic agents either as monotherapy or in combination. While great benefits of novel immunotherapies in oncology are evident, the safety of these therapies has to also be addressed as their desired pharmacology, immune activation, can lead to “exaggerated” effects and toxicity. This review is focused on the unique challenges of the nonclinical safety assessment of monoclonal antibodies that target immune checkpoint inhibitors and costimulatory molecules. This class of molecules represents several approved drugs and many more drug candidates in clinical development, for which significant experience has been gained. Their development illustrates challenges regarding the predictivity of the animal models for assessing safety and setting starting doses for first-in-human trials as well as the translatability of nonclinical in vitro and in vivo data to the human findings. Based on learnings from the experience to date, factors to consider and novel approaches to explore are discussed to help address the unique safety issues of immuno-oncology drug development.
Similar content being viewed by others
References
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Ponce R. Adverse consequences of immunostimulation. J Immunotoxicol. 2008;5(1):33–41. https://doi.org/10.1080/15476910801897920.
Gribble EJ, Sivakumar PV, Ponce RA, Hughes SD. Toxicity as a result of immunostimulation by biologics. Expert Opin Drug Metab Toxicol. 2007;3(2):209–34. https://doi.org/10.1517/17425255.3.2.209.
Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes J, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–28. https://doi.org/10.1056/NEJMoa063842.
Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010;161(3):512–26. https://doi.org/10.1111/j.1476-5381.2010.00922.x.
EMEA/CHMP/SWP/294648/. Guideline on strategies to identify and mitigate risks for first-in-man human clinical trials with investigational medicinal products. 2007.
ICH S9. Nonclinical evaluation for anticancer pharmaceuticals. March 2010. www.ich.org
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640–55. https://doi.org/10.1053/j.seminoncol.2015.05.014.
Hellmann MD, Friedman CF, Wolchok JD. Combinatorial cancer immunotherapies. Adv Immunol. 2016;130:251–77. https://doi.org/10.1016/bs.ai.2015.12.005.
Saber H, Gudi R, Manning M, Wearne E, Leighton JK. An FDA oncology analysis of immune activating products and first-in-human dose selection. Regul Toxicol Pharmacol. 2016;81:448–56. https://doi.org/10.1016/j.yrtph.2016.10.002.
Segal NH, Logan TF, Hodi S, McDermott D, Meleros I, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2016;23(8):1929–36. https://doi.org/10.1158/1078-0432.CCR-16-1272.
Melero I, et al. A phase I study of the safety, tolerability, pharmacokinetics, and immunoregulatory activity of urelumab (BMS-663513) in subjects with advanced and/or metastatic solid tumors and relapsed/refractory B-cell non-Hodgkin’s lymphoma (B-NHL) [abstract]. J Clin Oncol. 2013;31(Suppl):TPS3107.
Sznol M, et al. Phase I study of BMS-663513 a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) [abstract]. J Clin Oncol. 2008;26(Suppl):a3007.
Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37(5):508–16. https://doi.org/10.1053/j.seminoncol.2010.09.008.
Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178(7):4194–213. https://doi.org/10.4049/jimmunol.178.7.4194.
Wang M, Baumgart BR, Simutis F, Freebern W, Chadwick K, Bunch RT, Ju C, and Price K. Understanding anti-CD137-induced liver toxicity. In: The Toxicologist: Supplement to Toxicological Sciences, 150 (1), Society of Toxicology, 2016. Abstract no.1323.
Murphy JT, Burey AP, Beebe AM, Gu D, Presta LG, Merghoub T, et al. Anaphylaxis caused by repetitive doses of a GITR agonist monoclonal antibody in mice. Blood. 2014;123(14):2172–80. https://doi.org/10.1182/blood-2013-12-544742.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. https://doi.org/10.1016/1074-7613(95)90125-6.
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–8. https://doi.org/10.1126/science.270.5238.985.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. https://doi.org/10.1016/S1074-7613(00)80089-8.
Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20(3):327–36. https://doi.org/10.1016/S1074-7613(04)00050-0.
Nishimura H, Taku Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. https://doi.org/10.1126/science.291.5502.319.
Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–52. https://doi.org/10.1093/intimm/dxq026.
Ansari MJ1, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH: The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med, 2003; 198(1):63–69.
Miyazaki T, Dierich A, Benoist C, Mathis D. LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int Immunol. 1996;8(5):725–9. https://doi.org/10.1093/intimm/8.5.725.
Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, et al. Accelerated autoimmune diabetes in the absence of LAG-3. J Immunol. 2011;187(7):3493–8. https://doi.org/10.4049/jimmunol.1100714.
Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–83. https://doi.org/10.1200/JCO.2006.08.3311.
Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2(1):e23033. https://doi.org/10.4161/onci.23033.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–6. https://doi.org/10.1126/science.1198443.
Burris HA, Infante JR, Ansell SM, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J Clin Oncol. 2017;35:1–18.
Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–7. https://doi.org/10.1073/pnas.0830997100.
Price KD, Rao GK. Biological therapies for cancer. In: Plitnick LM, Herzyk DJ, editors. Nonclinical development of novel biologics, biosimilars, vaccines and specialty biologics. Elsevier Inc.: USA; 2013. p. 303–42. https://doi.org/10.1016/B978-0-12-394810-6.00013-7.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2015;25:9543–53.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219–42. https://doi.org/10.1111/j.1600-065X.2010.00923.x.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. https://doi.org/10.1056/NEJMoa1200690.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. https://doi.org/10.1056/NEJMoa1504030.
Ribas A, Hanson DC, Noe DA, Millham R, Guyot DJ, Bernstein SH, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12(7):873–83. https://doi.org/10.1634/theoncologist.12-7-873.
Price KP, Simutis F, Fletcher A, Ramaiah L, et al. Nonclinical safety evaluation of two distinct second generation variants of anti-CTLA4 monoclonal antibody, ipilimumab, in monkeys. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2017 Oct 26–30; Philadelphia, PA. Philadelphia (PA): AACR; Mol Cancer Ther, 2018;17(1 Suppl):Abstract nr LB-B33.
Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–56. https://doi.org/10.1158/2326-6066.CIR-14-0040.
Center for Drug Evaluation and Research Application Number: 125554 Orig1s000, Summary Review for Nivolumab. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125554Orig1s000SumR.pdf . 2014; Accessed 24 July 2017.
Center for Drug Evaluation and Research Application Number: 125514 Orig1s000, Summary Review for Pembrolizumab. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125514Orig1s000SumR.pdf . 2014; Accessed 24 July 2017.
Stewart RS, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunother Cancer. 2014;2(1):29–38. https://doi.org/10.1186/s40425-014-0029-x.
Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19(1):101–13. https://doi.org/10.1016/j.ccr.2010.11.012.
Brennan FR, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. Monoclon Antibodies. 2010;2:233e255.
Spigel D. et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) [ASCO abstract 8008]. J Clin Oncol, 2013; 31(15)(suppl).
Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–710. https://doi.org/10.1084/jem.20130579.
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22. https://doi.org/10.1200/JCO.2012.44.6112.
Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–9. https://doi.org/10.1158/1078-0432.CCR-08-0783.
Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333(6045):1030–4. https://doi.org/10.1126/science.1206954.
White AL, Chan HTC, Roghanian A, French RR, Mockridge CI, Tutt AL, et al. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187(4):1754–63. https://doi.org/10.4049/jimmunol.1101135.
Ravetch JV and Nimmerjahn F. Fc receptors. In: Paul WE, editor. Fundamental Immunology. Lippincott-Raven; 2008. p. 684–705.
Morris NP, Peters C, Montler R, Hu HM, Curti BD, Urba WJ, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44(12):3112–21. https://doi.org/10.1016/j.molimm.2007.02.004.
Vitale LA, He LZ, Thomas LJ, Widger J, Weidlick J, Crocker A, et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012;18(14):3812–21. https://doi.org/10.1158/1078-0432.CCR-11-3308.
Sukumar S, Wilson DC, Yu Ying et al. Characterization of MK-4166, a clinical agonistic mAb that targets human GITR and inhibits the generation and activity of Tregs. Cancer Res; 2017. Published OnlineFirst on June 13, 2017; https://doi.org/10.1158/0008-5472.CAN-16-1439.
Keler T, Halk E, Vitale L, O’Neill T, Blanset D, Lee S, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171(11):6251–9. https://doi.org/10.4049/jimmunol.171.11.6251.
Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell. 2016;29(6):820–31. https://doi.org/10.1016/j.ccell.2016.05.001.
Semple KM and Howard KE. Human Fc receptor expression in immune humanized mice. In: The Toxicologist: Supplement to Toxicological Sciences, 156 (1), Society of Toxicology; 2017. Abstract no. 2550.
Howard KE, Zadrozny L, Semple KM, Shea K, and Weaver JL. Autoimmunity induced by ipilimumab in immune humanized mice. In: The Toxicologist: Supplement to Toxicological Sciences, 156 (1), Society of Toxicology; 2017. Abstract no. 1693.
Howard KE, Zadrozny L, and Weaver JL. Immune humanized mouse model: autoimmunity induced by nivolumab. American College of Toxicology; 2016. Abstract no. P516. www.actox.org
Morrissey KM, Yuraszeck TM, Li C-C, Zhang Y, Kasichayala S. Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin Transl Sci. 2016;9(2):89–104. https://doi.org/10.1111/cts.12391.
Selby MJ, Engelhardt JJ, Johnston RJ, LS L, Han M, Thudium K, et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One. 2016;11(9):e0161779. https://doi.org/10.1371/journal.pone.0161779.
ICH S9 Guideline on nonclinical evaluation for anticancer pharmaceuticals - Qestions and Answers; September 2016 (Step 2).
ICH S5(R2). Detection of toxicity to reproduction for medicinal products and toxicity to male fertility. Addendum dated 9 November 2000, incorporated in November 2005. www.ich.org
ICH S6(R1). Preclinical safety evaluation of biotechnology-derived pharmaceuticals. Parent Guideline dated 16 July 1997, Addendum dated 12 June 2011, incorporated in June 2011. www.ich.org
Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141(6):715–24. https://doi.org/10.1530/REP-10-0360.
Aluvihare VR, Kallikourdis M, Betz AG, Regulatory T. Cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71. https://doi.org/10.1038/ni1037.
Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231–7. https://doi.org/10.1084/jem.20050019.
Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Zambon Bertoja A, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–22. https://doi.org/10.1016/S0002-9440(10)62302-4.
Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon Bertoja A, Fest S, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006;36(1):82–94. https://doi.org/10.1002/eji.200535428.
Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. https://doi.org/10.1111/j.1365-2567.2004.01869.x.
Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345–51. https://doi.org/10.4049/jimmunol.178.6.3345.
Kaufman KA, Bowen JA, Tsai AF, Bluestone JA, Hunt JS, Ober C. The CTLA-4 gene is expressed in placental fibroblasts. Mol Hum Reprod. 1999;5(1):84–7. https://doi.org/10.1093/molehr/5.1.84.
Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, et al. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-ɣ increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod. 2005;11(12):865–70. https://doi.org/10.1093/molehr/gah246.
Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80(1-2):12–21. https://doi.org/10.1016/j.jri.2008.12.001.
Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk H-D, et al. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol. 2009;62(5):283–92. https://doi.org/10.1111/j.1600-0897.2009.00737.x.
Poulet FM, Wolf JJ, Herzyk DJ, DeGeorge JJ. An evaluation of the impact of PD-1 pathway blockade on reproductive safety of therapeutic PD-1 inhibitors. Birth Defects Res. 2016;107(2):108–19. https://doi.org/10.1002/bdrb.21176.
Prell RA, Halpern WG, Rao GK. Perspective on a modified developmental and reproductive toxicity testing strategy for cancer immunotherapy. Int J Toxicol. 2016;35(3):263–7. https://doi.org/10.1177/1091581815625596.
Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR-GITRL system, a novel player in shock and inflammation. Sci World J. 2007;7:533–66. https://doi.org/10.1100/tsw.2007.106.
Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Investig. 2007;117(5):1399–411. https://doi.org/10.1172/JCI28214.
Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179(8):5211–9. https://doi.org/10.4049/jimmunol.179.8.5211.
Erlebacher A. Immunology of the maternal-fetal Interface. Annu Rev Immunol. 2013;31(1):387–411. https://doi.org/10.1146/annurev-immunol-032712-100003.
Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–6. https://doi.org/10.1038/nature11462.
Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. https://doi.org/10.1016/j.cell.2012.05.031.
Cheng S-B, Sharma S. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol. 2015;73(6):487–500. https://doi.org/10.1111/aji.12329.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Mohammad Tabrizifard and Vaishnavi Ganti
Rights and permissions
About this article
Cite this article
Herzyk, D.J., Haggerty, H.G. Cancer Immunotherapy: Factors Important for the Evaluation of Safety in Nonclinical Studies. AAPS J 20, 28 (2018). https://doi.org/10.1208/s12248-017-0184-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-017-0184-3