ABSTRACT
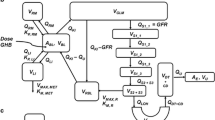
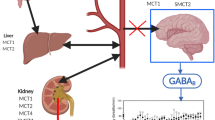
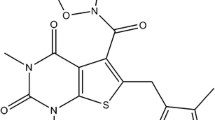
The drug of abuse γ-hydroxybutyric acid (GHB) demonstrates complex toxicokinetics with dose-dependent metabolic and renal clearance. GHB is a substrate of monocarboxylate transporters (MCTs) which are responsible for the saturable renal reabsorption of GHB. MCT expression is observed in many tissues and therefore may impact the tissue distribution of GHB. The objective of the present study was to evaluate the tissue distribution kinetics of GHB at supratherapeutic doses. GHB (400, 600, and 800 mg/kg iv) or GHB 600 mg/kg plus l-lactate (330 mg/kg iv bolus followed by 121 mg/kg/h infusion) was administered to rats and blood and tissues were collected for up to 330 min post-dose. Kp values for GHB varied in both a tissue- and dose-dependent manner and were less than 0.5 (except in the kidney). Nonlinear partitioning was observed in the liver (0.06 at 400 mg/kg to 0.30 at 800 mg/kg), kidney (0.62 at 400 mg/kg to 0.98 at 800 mg/kg), and heart (0.15 at 400 mg/kg to 0.29 at 800 mg/kg), with Kp values increasing with dose consistent with saturation of transporter-mediated efflux. In contrast, lung partitioning decreased in a dose-dependent manner (0.43 at 400 mg/kg to 0.25 at 800 mg/kg) suggesting saturation of active uptake. l-lactate administration decreased Kp values in liver, striatum, and hippocampus and increased Kp values in lung and spleen. GHB demonstrates tissue-specific nonlinear distribution consistent with the involvement of monocarboxylate transporters. These observed complexities are likely due to the involvement of MCT1 and 4 with different affinities and directionality for GHB transport.





Similar content being viewed by others
REFERENCES
Gold BI, Roth RH. Kinetics of in vivo conversion of gamma-[3H]aminobutyric acid to gamma-[3H]hydroxybutyric acid by rat brain. J Neurochem. 1977;28(5):1069–73. https://doi.org/10.1111/j.1471-4159.1977.tb10670.x.
Snead OC III, Liu CC. Gamma-hydroxybutyric acid binding sites in rat and human brain synaptosomal membranes. Biochem Pharmacol. 1984;33(16):2587–90. https://doi.org/10.1016/0006-2952(84)90629-4.
Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, et al. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB). FASEB J. 2003;17(12):1691–3. https://doi.org/10.1096/fj.02-0846fje.
Andriamampandry C, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB J. 2007;21(3):885–95. https://doi.org/10.1096/fj.06-6509com.
Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51(3):337–61. https://doi.org/10.1016/S0301-0082(96)00064-0.
Morse BL, Vijay N, Morris ME. gamma-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol Pharmacol. 2012;82(2):226–35. https://doi.org/10.1124/mol.112.078154.
Gallimberti L, Spella MR, Soncini CA, Gessa GL. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol. 2000;20(3):257–62. https://doi.org/10.1016/S0741-8329(99)00089-0.
Okun MS, Boothby LA, Bartfield RB, Doering PL. GHB: an important pharmacologic and clinical update. J Pharm Pharm Sci. 2001;4(2):167–75.
Wong CG, Gibson KM, Snead OC III. From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25(1):29–34. https://doi.org/10.1016/j.tips.2003.11.001.
Carter LP, Koek W, France CP. Behavioral analyses of GHB: receptor mechanisms. Pharmacol Ther. 2009;121(1):100–14. https://doi.org/10.1016/j.pharmthera.2008.10.003.
Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, et al. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45(4):353–6.
Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208(1):7–11.
Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69(3):356–8. https://doi.org/10.1002/jps.2600690331.
Morris ME, Hu K, Wang Q. Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther. 2005;313(3):1194–202. https://doi.org/10.1124/jpet.105.083253.
Felmlee MA, Wang Q, Cui D, Roiko SA, Morris ME. Mechanistic toxicokinetic model for gamma-hydroxybutyric acid: inhibition of active renal reabsorption as a potential therapeutic strategy. AAPS J. 2010;12(3):407–16. https://doi.org/10.1208/s12248-010-9197-x.
Wang Q, Lu Y, Yuan M, Darling IM, Repasky EA, Morris ME. Characterization of monocarboxylate transport in human kidney HK-2 cells. Mol Pharm. 2006;3(6):675–85. https://doi.org/10.1021/mp060037b.
Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008;10(2):311–21. https://doi.org/10.1208/s12248-008-9035-6.
Lam WK, Felmlee MA, Morris ME. Monocarboxylate transporter-mediated transport of gamma-hydroxybutyric acid in human intestinal Caco-2 cells. Drug Metab Dispos. 2010;38(3):441–7. https://doi.org/10.1124/dmd.109.030775.
Benavides J, Rumigny JF, Bourguignon JJ, Wermuth CG, Mandel P, Maitre M. A high-affinity, Na+-dependent uptake system for gamma-hydroxybutyrate in membrane vesicles prepared from rat brain. J Neurochem. 1982;38(6):1570–5. https://doi.org/10.1111/j.1471-4159.1982.tb06634.x.
Bhattacharya I, Boje KM. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther. 2004;311(1):92–8. https://doi.org/10.1124/jpet.104.069682.
Roiko SA, Felmlee MA, Morris ME. Brain uptake of the drug of abuse gamma-hydroxybutyric acid in rats. Drug Metab Dispos. 2012;40(1):212–8. https://doi.org/10.1124/dmd.111.041749.
Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121(1):29–40. https://doi.org/10.1016/j.pharmthera.2008.09.005.
Felmlee MA, Roiko SA, Morse BL, Morris ME. Concentration-effect relationships for the drug of abuse gamma-hydroxybutyric acid. J Pharmacol Exp Ther. 2010;333(3):764–71. https://doi.org/10.1124/jpet.109.165381.
Wang Q, Wang X, Morris ME. Effects of L-lactate and D-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab Dispos. 2008;36(11):2244–51. https://doi.org/10.1124/dmd.108.022996.
Morse BL, Vijay N, Morris ME. Mechanistic modeling of monocarboxylate transporter-mediated toxicokinetic/toxicodynamic interactions between gamma-hydroxybutyrate and L-lactate. AAPS J. 2014;16(4):756–70. https://doi.org/10.1208/s12248-014-9593-8.
Vijay N, Morse BL, Morris ME. A novel monocarboxylate transporter inhibitor as a potential treatment strategy for gamma-hydroxybutyric acid overdose. Pharm Res. 2015;32(6):1894–906. https://doi.org/10.1007/s11095-014-1583-0.
Wang Q, Morris ME. The role of monocarboxylate transporter 2 and 4 in the transport of gamma-hydroxybutyric acid in mammalian cells. Drug Metab Dispos. 2007;35(8):1393–9. https://doi.org/10.1124/dmd.107.014852.
Wang Q, Darling IM, Morris ME. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: role of monocarboxylate transporters. J Pharmacol Exp Ther. 2006;318(2):751–61. https://doi.org/10.1124/jpet.106.105965.
Cui D, Morris ME. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): characterization of SMCT-mediated uptake and inhibition. Drug Metab Dispos. 2009;37(7):1404–10. https://doi.org/10.1124/dmd.109.027169.
Morse BL, Felmlee MA, Morris ME. gamma-Hydroxybutyrate blood/plasma partitioning: effect of physiologic pH on transport by monocarboxylate transporters. Drug Metab Dispos. 2012;40(1):64–9. https://doi.org/10.1124/dmd.111.041285.
Khor SP, Bozigian H, Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. II. Distribution of phencyclidine in the rat. Drug Metab Dispos. 1991;19(2):486–90.
Triplett JW, Hayden TL, McWhorter LK, Gautam SR, Kim EE, Bourne DW. Determination of gallium concentration in "blood-free" tissues using a radiolabeled blood marker. J Pharm Sci. 1985;74(9):1007–9. https://doi.org/10.1002/jps.2600740922.
Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16(3):303–9. https://doi.org/10.1007/BF01062139.
Wang X, Wang Q, Morris ME. Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: potential involvement of monocarboxylate transporters. AAPS J. 2008;10(1):47–55. https://doi.org/10.1208/s12248-007-9001-8.
Wang Q, Lu Y, Morris ME. Monocarboxylate transporter (MCT) mediates the transport of gamma-hydroxybutyrate in human kidney HK-2 cells. Pharm Res. 2007;24(6):1067–78. https://doi.org/10.1007/s11095-006-9228-6.
Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Phys. 1993;264(4 Pt 1):C761–82.
Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10(1):193–9. https://doi.org/10.1208/s12248-008-9022-y.
Juel C, Halestrap AP. Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J Physiol. 1999;517(Pt 3):633–42. https://doi.org/10.1111/j.1469-7793.1999.0633s.x.
Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–27. https://doi.org/10.1042/bj3500219.
Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, et al. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem. 1998;273(26):15920–6. https://doi.org/10.1074/jbc.273.26.15920.
Roiko SA, Vijay N, Felmlee MA, Morris ME. Brain extracellular gamma-hydroxybutyrate concentrations are decreased by L-lactate in rats: role in the treatment of overdoses. Pharm Res. 2013;30(5):1338–48. https://doi.org/10.1007/s11095-013-0973-z.
Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J Physiol. 2004;559(Pt 1):85–101. https://doi.org/10.1113/jphysiol.2004.067793.
Morris ME, Morse BL, Baciewicz GJ, Tessena MM, Acquisto NM, Hutchinson DJ, et al. Monocarboxylate transporter inhibition with osmotic diuresis increases gamma-hydroxybutyrate renal elimination in humans: a proof-of-concept study. Journal of clinical toxicology. 2011;1(2):1000105. https://doi.org/10.4172/2161-0495.1000105.
Morris ME, Rodriguez-Cruz V, Felmlee MA. SLC and ABC transporters: expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J. 2017;19(5):1317–31. https://doi.org/10.1208/s12248-017-0110-8.
Pierre K, Parent A, Jayet PY, Halestrap AP, Scherrer U, Pellerin L. Enhanced expression of three monocarboxylate transporter isoforms in the brain of obese mice. J Physiol. 2007;583(Pt 2):469–86. https://doi.org/10.1113/jphysiol.2007.138594.
Goncalves P, Araujo JR, Pinho MJ, Martel F. Modulation of butyrate transport in Caco-2 cells. Naunyn Schmiedeberg's Arch Pharmacol. 2009;379(4):325–36. https://doi.org/10.1007/s00210-008-0372-x.
Morse BL, Chadha GS, Felmlee MA, Follman KE, Morris ME. Effect of chronic gamma-hydroxybutyrate (GHB) administration on GHB toxicokinetics and GHB-induced respiratory depression. Am J Drug Alcohol Abuse. 2017;43(6):1–8.
Cupeiro R, Gonzalez-Lamuno D, Amigo T, Peinado AB, Ruiz JR, Ortega FB, et al. Influence of the MCT1-T1470A polymorphism (rs1049434) on blood lactate accumulation during different circuit weight trainings in men and women. J Sci Med Sport. 2012;15(6):541–7. https://doi.org/10.1016/j.jsams.2012.03.009.
Fei F, Guo X, Chen Y, Liu X, Tu J, Xing J, et al. Polymorphisms of monocarboxylate transporter genes are associated with clinical outcomes in patients with colorectal cancer. J Cancer Res Clin Oncol. 2015;141(6):1095–102.
Lyon RC, Johnston SM, Panopoulos A, Alzeer S, McGarvie G, Ellis EM. Enzymes involved in the metabolism of gamma-hydroxybutyrate in SH-SY5Y cells: identification of an iron-dependent alcohol dehydrogenase ADHFe1. Chem Biol Interact. 2009;178(1–3):283–7. https://doi.org/10.1016/j.cbi.2008.10.025.
O'Connor T, Ireland LS, Harrison DJ, Hayes JD. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem J. 1999;343(Pt 2):487–504. https://doi.org/10.1042/bj3430487.
Malaspina P, Picklo MJ, Jakobs C, Snead OC, Gibson KM. Comparative genomics of aldehyde dehydrogenase 5a1 (succinate semialdehyde dehydrogenase) and accumulation of gamma-hydroxybutyrate associated with its deficiency. Hum Genomics. 2009;3(2):106–20. https://doi.org/10.1186/1479-7364-3-2-106.
Alzeer S, Ellis EM. Metabolism of gamma hydroxybutyrate in human hepatoma HepG2 cells by the aldo-keto reductase AKR1A1. Biochem Pharmacol. 2014;92(3):499–505. https://doi.org/10.1016/j.bcp.2014.09.004.
ACKNOWLEDGEMENTS
The study received grant support from the NIH (R01 DA023223).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felmlee, M.A., Morse, B.L., Follman, K.E. et al. The Drug of Abuse Gamma-Hydroxybutyric Acid Exhibits Tissue-Specific Nonlinear Distribution. AAPS J 20, 21 (2018). https://doi.org/10.1208/s12248-017-0180-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-017-0180-7