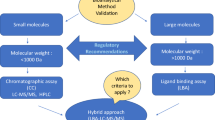
Abstract
The L1 Global Harmonization Team provides recommendations specifically for run acceptance of ligand binding methods used in bioanalysis of macromolecules in support of pharmacokinetics. The team focused on standard curve calibrators and quality controls for use in both pre-study validation and in-study sample analysis, including their preparation and acceptance criteria. The team also considered standard curve editing and the concept of total error.
Similar content being viewed by others
References
European Medicines Agency, Committee for Medicinal Products for Human Use. Guideline on bioanalytical method validation. July 2011.
Health Products and Food Branch. Guidance Document: Non-clinical laboratory study data supporting drug product applications and submissions: adherence to Good Laboratory Practice. Ottawa, ON, Canada (2010).
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Guidance for industry: bioanalytical method validation. Federal Register. 2001;66:28526–28527.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) March 2003. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf.
DeSilva B, Smith W, Weiner R. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20:1885–900.
Kelley M, DeSilva B. Key elements of bioanalytical method validation for macromolecules. AAPS J. 2007;9:E156–63.
Findlay JWA, Dillard RF. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 2007;9(2):E260–7.
DeSilva B et al. 2012 white paper on recent issues in bioanalysis and regulatory agencies' alignment towards multiple harmonized bioanalytical guidance/guidelines. Bioanalysis. 2012;4(18):2213–26.
Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J of Pharm Biomed Anal. 2000;21(6):1249–73.
OECD series on principles of good laboratory practice and compliance Monitoring. Number 1. OECD principles on good laboratory practice (as revised in 1997). 1–39 (1998).
Ministry of Health and Welfare (Japan) Notification No. 443, Guidance for toxicokinetics, July, 1996.
Japan Pharmaceutical Administration and Regulations. Information in English on Japanese regulatory affairs, March, 2012. http://www.jpma.or.jp/english/parj/1003.html.
Ministry of Health and Welfare (Japan) Ordinance No. 21, Ordinance on the GLP Standard for Conduct of Nonclinical Safety Studies of Drugs, March, 1997.
State Food and Drug Administration (China) Guide for the research of human bioavailability and bioequivalence about chemical drug, March, 2005.
Ministry of Health. National Agency for Sanitary Vigilance—Anvisa. Minimum requirements for the validation of bioanalytical methods used in studies with the purpose of registration and post-registration of medicines. RDC No. 27, May 2012.
Ministry of Health. National Agency for Sanitary Vigilance—Anvisa. Guide for the preparation of technical report on bioavailability/bioequivalence. RE No. 895, May 2003.
Australian Government, Department of Health and Ageing Therapeutic Goods Administration. Australian Regulatory Guidelines for Prescription Medicines, June 2004.
Acknowledgments
The team would like to thank Drs. Ronald Bowsher and Binodh DeSilva for their contributions to the discussions on run acceptance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Binodh DeSilva and Philip Timmerman
Rights and permissions
About this article
Cite this article
Kelley, M., Beaver, C., Stevenson, L.F. et al. Large Molecule Run Acceptance: Recommendation for Best Practices and Harmonization from the Global Bioanalysis Consortium Harmonization Team. AAPS J 16, 221–225 (2014). https://doi.org/10.1208/s12248-013-9553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9553-8