Abstract
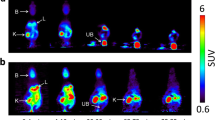
Glyburide (glibenclamide, GLB) is a widely prescribed antidiabetic with potential beneficial effects in central nervous system injury and diseases. In vitro studies show that GLB is a substrate of organic anion transporting polypeptide (OATP) and ATP-binding cassette (ABC) transporter families, which may influence GLB distribution and pharmacokinetics in vivo. In the present study, we used [11C]GLB positron emission tomography (PET) imaging to non-invasively observe the distribution of GLB at a non-saturating tracer dose in baboons. The role of OATP and P-glycoprotein (P-gp) in [11C]GLB whole-body distribution, plasma kinetics, and metabolism was assessed using the OATP inhibitor rifampicin and the dual OATP/P-gp inhibitor cyclosporine. Finally, we used in situ brain perfusion in mice to pinpoint the effect of ABC transporters on GLB transport at the blood–brain barrier (BBB). PET revealed the critical role of OATP on liver [11C]GLB uptake and its subsequent impact on [11C]GLB metabolism and plasma clearance. OATP-mediated uptake also occurred in the myocardium and kidney parenchyma but not the brain. The inhibition of P-gp in addition to OATP did not further influence [11C]GLB tissue and plasma kinetics. At the BBB, the inhibition of both P-gp and breast cancer resistance protein (BCRP) was necessary to demonstrate the role of ABC transporters in limiting GLB brain uptake. This study demonstrates that GLB distribution, metabolism, and elimination are greatly dependent on OATP activity, the first step in GLB hepatic clearance. Conversely, P-gp, BCRP, and probably multidrug resistance protein 4 work in synergy to limit GLB brain uptake.





Similar content being viewed by others
References
Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2012;18:1805–11.
Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32:1699–717.
Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27:1096–102.
Cygalova LH, Hofman J, Ceckova M, Staud F. Transplacental pharmacokinetics of glyburide, rhodamine 123, and BODIPY FL prazosin: effect of drug efflux transporters and lipid solubility. J Pharmacol Exp Ther. 2009;331:1118–25.
Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol. 2008;73:949–59.
Pollex E, Lubetsky A, Koren G. The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta. Placenta. 2008;29:743–7.
Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GDV, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010;202:383.e1–7.
Gedeon C, Anger G, Piquette-Miller M, Koren G. Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta. 2008;29:39–43.
Golstein PE, Boom A, van Geffel J, Jacobs P, Masereel B, Beauwens R. P-glycoprotein inhibition by glibenclamide and related compounds. Pflugers Arch. 1999;437:652–60.
Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–23.
Koenen A, Köck K, Keiser M, Siegmund W, Kroemer HK, Grube M. Steroid hormones specifically modify the activity of organic anion transporting polypeptides. Eur J Pharm Sci. 2012;47:774–80.
König J, Müller F, Fromm MF. Transporters and drug–drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–66.
Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–72.
Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin's inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin Pharmacol Ther. 2009;85:78–85.
Shawahna R, Uchida Y, Declèves X, Ohtsuki S, Yousif S, Dauchy S, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–41.
Agarwal S, Uchida Y, Mittapalli RK, Sane R, Terasaki T, Elmquist WF. Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metab Dispos. 2012;40:1164–9.
Kuhnast B, Damont A, Tournier N, Saba W, Valette H, Bottlaender M, et al. Radiosynthesis of [C-11]glyburide for in vivo imaging of BCRP function with PET. J Label Compounds Radiopharm. 2011;54 Suppl 1:S262.
Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann J-M, Declèves X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int J Neuropsychopharmacol. 2010;13:905–15.
Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann J-M. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood–brain barrier. Cancer Res. 2004;64:3296–301.
Sane R, Agarwal S, Mittapalli RK, Elmquist WF. Saturable active efflux by P-glycoprotein and breast cancer resistance protein at the blood–brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J Pharmacol Exp Ther. 2013;345:111–24.
Cattelotte J, André P, Ouellet M, Bourasset F, Scherrmann J-M, Cisternino S. In situ mouse carotid perfusion model: glucose and cholesterol transport in the eye and brain. J Cereb Blood Flow Metab. 2008;28:1449–59.
Mease K, Sane R, Podila L, Taub ME. Differential selectivity of efflux transporter inhibitors in Caco-2 and MDCK-MDR1 monolayers: a strategy to assess the interaction of a new chemical entity with P-gp, BCRP, and MRP2. J Pharm Sci. 2012;101:1888–97.
Xie M, Rich TC, Scheitrum C, Conti M, Richter W. Inactivation of multidrug resistance proteins disrupts both cellular extrusion and intracellular degradation of cAMP. Mol Pharmacol. 2011;80:281–93.
Zhou L, Naraharisetti SB, Liu L, Wang H, Lin YS, Isoherranen N, et al. Contributions of human cytochrome P450 enzymes to glyburide metabolism. Biopharm Drug Dispos. 2010;31:228–42.
Amundsen R, Åsberg A, Ohm IK, Christensen H. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos. 2012;40:655–61.
Li X-Q, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–7.
Lau YY, Okochi H, Huang Y, Benet LZ. Pharmacokinetics of atorvastatin and its hydroxy metabolites in rats and the effects of concomitant rifampicin single doses: relevance of first-pass effect from hepatic uptake transporters, and intestinal and hepatic metabolism. Drug Metab Dispos. 2006;34:1175–81.
De Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–9.
Lin F, Marchetti S, Pluim D, Iusuf D, Mazzanti R, Schellens JHM, et al. Abcc4 together with Abcb1 and Abcg2 form a robust co-operative drug efflux system that restricts the brain entry of camptothecin analogs. Clin Cancer Res. 2013;19:2084–95.
Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, et al. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug–drug interactions. J Med Chem. 2012;55:4740–63.
Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–82.
Pearson JG, Antal EJ, Raehl CL, Gorsch HK, Craig WA, Albert KS, et al. Pharmacokinetic disposition of 14C-glyburide in patients with varying renal function. Clin Pharmacol Ther. 1986;39:318–24.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2008;23:22–44.
Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006;80:607–20.
Juurlink DN, Gomes T, Shah BR, Mamdani MM. Adverse cardiovascular events during treatment with glyburide (glibenclamide) or gliclazide in a high-risk population. Diabet Med. 2012;29:1524–8.
Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood–brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013. doi:10.1002/jps.23575.
Clark DE. In silico prediction of blood–brain barrier permeation. Drug Discov Today. 2003;8:927–33.
Nanovskaya TN, Patrikeeva S, Hemauer S, Fokina V, Mattison D, Hankins GD, et al. Effect of albumin on transplacental transfer and distribution of rosiglitazone and glyburide. J Matern Fetal Neonatal Med. 2008;21:197–207.
Takashima T, Kitamura S, Wada Y, Tanaka M, Shigihara Y, Ishii H, et al. PET imaging-based evaluation of hepatobiliary transport in humans with (15R)-11C-TIC-Me. J Nucl Med. 2012;53:741–8.
Leonhardt M, Keiser M, Oswald S, Kühn J, Jia J, Grube M, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–8.
Bruderer S, Aänismaa P, Homery M-C, Häusler S, Landskroner K, Sidharta PN, et al. Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist. AAPS J. 2012;14:68–78.
De Bruyn T, Fattah S, Stieger B, Augustijns P, Annaert P. Sodium fluorescein is a probe substrate for hepatic drug transport mediated by OATP1B1 and OATP1B3. J Pharm Sci. 2011;100:5018–30.
Picard N, Levoir L, Lamoureux F, Yee SW, Giacomini KM, Marquet P. Interaction of sirolimus and everolimus with hepatic and intestinal organic anion-transporting polypeptide transporters. Xenobiotica. 2011;41:752–7.
König J, Glaeser H, Keiser M, Mandery K, Klotz U, Fromm MF. Role of organic anion-transporting polypeptides for cellular mesalazine (5-aminosalicylic acid) uptake. Drug Metab Dispos. 2011;39:1097–102.
Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–51.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–77.
ACKNOWLEDGMENTS
We thank Maria Smirnova, Vincent Brulon, and Amandine Grelier, who contributed to the research. Statistical analyses were performed by Dr. Marcel Debray. The English text was edited by Dr. S. Rasika. Salvatore Cisternino received a grant from the Commissariat à l'énergie atomique et aux énergies alternatives and the Assistance Publique des Hôpitaux de Paris.
Conflict of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tournier, N., Saba, W., Cisternino, S. et al. Effects of Selected OATP and/or ABC Transporter Inhibitors on the Brain and Whole-Body Distribution of Glyburide. AAPS J 15, 1082–1090 (2013). https://doi.org/10.1208/s12248-013-9514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9514-2