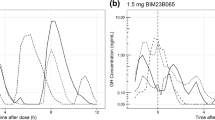
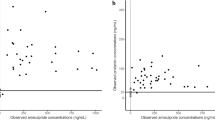
Abstract
Prolactin elevation is a side effect of all currently available D2 receptor antagonists used in the treatment of schizophrenia. Prolactin elevation is the result of a direct antagonistic D2 effect blocking the tonic inhibition of prolactin release by dopamine. The aims of this work were to assess the correlation between in vitro estimates of D2 receptor affinity and pharmacokinetic–pharmacodynamic model-based estimates obtained from analysis of clinical data using an agonist–antagonist interaction (AAI) model and to assess the value of such a correlation in early prediction of full prolactin time profiles. A population model describing longitudinal prolactin data was fitted to clinical data from 16 clinical phases 1 and 3 trials including five different compounds. Pharmacokinetic data were modeled for each compound and the prolactin model was both fitted in per-compound fits as well as simultaneously to all prolactin data. Estimates of prolactin elevating potency were compared to corresponding in vitro values and their predictability was evaluated through model-based simulations. The model successfully described the prolactin time course for all compounds. Estimates derived from experimental preclinical data and the model fit of the clinical data were strongly correlated (p < 0.001), and simulations adequately predicted the prolactin elevation in five out of six compounds. The AAI model has the potential to be used in drug development to predict prolactin response for a given exposure of D2 antagonists using routinely produced preclinical data.





Similar content being viewed by others
References
Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50(11):873–83.
Biedermann F, Fleischhacker WW. Emerging drugs for schizophrenia. Expert Opin Emerg Drugs. 2011;16(2):271–82.
Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–73.
Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry. 2002;159(1):133–5.
Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251(1):238–46.
Kapur S, Langlois X, Vinken P, Megens AA, De Coster R, Andrews JS. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood–brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther. 2002;302(3):1129–34.
Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry. 2001;158(3):360–9.
Langer G, Sachar EJ, Gruen PH, Halpern FS. Human prolactin responses to neuroleptic drugs correlate with antischizophrenic potency. Nature. 1977;266(5603):639–40.
Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–9.
Bagli M, Suverkrup R, Quadflieg R, Hoflich G, Kasper S, Moller HJ, et al. Pharmacokinetic–pharmacodynamic modeling of tolerance to the prolactin-secreting effect of chlorprothixene after different modes of drug administration. J Pharmacol Exp Ther. 1999;291(2):547–54.
Friberg LE, Vermeulen AM, Petersson KJ, Karlsson MO. An agonist–antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409–17.
Movin-Osswald G, Hammarlund-Udenaes M. Prolactin release after remoxipride by an integrated pharmacokinetic–pharmacodynamic model with intra- and interindividual aspects. J Pharmacol Exp Ther. 1995;274(2):921–7.
Ma G, Friberg LE, Movin-Osswald G, Karlsson MO. Comparison of the agonist–antagonist interaction model and the pool model for the effect of remoxipride on prolactin. Br J Clin Pharmacol. 2010;70(6):815–24.
Te Beek ET, Moerland M, de Boer P, van Nueten L, de Kam ML, Burggraaf J, et al. Pharmacokinetics and central nervous system effects of the novel dopamine D2 receptor antagonist JNJ-37822681. J Psychopharmacol. 2011;26(8):1119–27.
Mesens N, Steemans M, Hansen E, Verheyen GR, Van Goethem F, Van Gompel J. Screening for phospholipidosis induced by central nervous drugs: comparing the predictivity of an in vitro assay to high throughput in silico assays. Toxicol In Vitro. 2010;24(5):1417–25.
Tresadern G, Bartolome JM, Macdonald GJ, Langlois X. Molecular properties affecting fast dissociation from the D2 receptor. Bioorg Med Chem. 2011;19(7):2231–41.
Movin-Osswald G, Hammarlund-Udenaes M. Remoxipride: pharmacokinetics and effect on plasma prolactin. Br J Clin Pharmacol. 1991;32(3):355–60.
Vermeulen A, Piotrovsky V, Ludwig EA. Population pharmacokinetics of risperidone and 9-hydroxyrisperidone in patients with acute episodes associated with bipolar I disorder. J Pharmacokinet Pharmacodyn. 2007;34(2):183–206.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Prog Biomed. 2004;75(2):85–94.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Prog Biomed. 1999;58(1):51–64.
R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010.
Friberg LE, Sandstrom M, Karlsson MO. Scaling the time-course of myelosuppression from rats to patients with a semi-physiological model. Invest New Drugs. 2010;28(6):744–53.
Zuideveld KP, Van der Graaf PH, Peletier LA, Danhof M. Allometric scaling of pharmacodynamic responses: application to 5-Ht1A receptor mediated responses from rat to man. Pharm Res. 2007;24(11):2031–9.
Yassen A, Olofsen E, Kan J, Dahan A, Danhof M. Animal-to-human extrapolation of the pharmacokinetic and pharmacodynamic properties of buprenorphine. Clin Pharmacokinet. 2007;46(5):433–47.
Lepist EI, Jusko WJ. Modeling and allometric scaling of s(+)-ketoprofen pharmacokinetics and pharmacodynamics: a retrospective analysis. J Vet Pharmacol Ther. 2004;27(4):211–8.
Raaflaub J. On the pharmacokinetics of chlorprothixene in man. Experientia. 1975;31(5):557–8.
Quitkin FM, editors. Current psychotherapeutic drugs. 2nd ed. Washington: American Psychiatric; 1998.
Seyffart G. Drug dosage in renal insufficiency. New York: Springer; 1991.
Schmidt ME, Kent JM, Daly E, Janssens L, Van Osselaer N, Husken G, et al. A double-blind, randomized, placebo-controlled study with JNJ-37822681, a novel, highly selective, fast dissociating D(2) receptor antagonist in the treatment of acute exacerbation of schizophrenia. Eur Neuropsychopharmacol. 2012;22(10):721–33.
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996;124(1–2):57–73.
Cooper DS, Ridgway EC, Kliman B, Kjellberg RN, Maloof F. Metabolic clearance and production rates of prolactin in man. J Clin Invest. 1979;64(6):1669–80.
Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35(3):151–63.
Rao ML, Gross G, Halaris A, Huber G, Marler M, Strebel B, et al. Hyperdopaminergia in schizophreniform psychosis: a chronobiological study. Psychiatry Res. 1993;47(2):187–203.
Acknowledgments
Klas Petersson would like to acknowledge Janssen Pharmaceutica N.V. for sponsorship of his PhD work. Part of the computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project p2011063
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersson, K.J., Vermeulen, A.M. & Friberg, L.E. Predictions of In Vivo Prolactin Levels from In Vitro K i Values of D2 Receptor Antagonists Using an Agonist–Antagonist Interaction Model. AAPS J 15, 533–541 (2013). https://doi.org/10.1208/s12248-012-9450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9450-6