Abstract
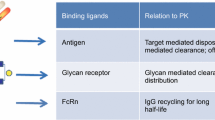
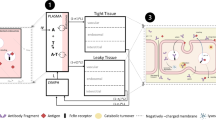
Monoclonal antibodies are increasingly being developed to treat multiple disease areas, including those related to oncology, immunology, neurology, and ophthalmology. There are multiple factors, such as charge, size, neonatal Fc receptor (FcRn) binding affinity, target affinity and biology, immunoglobulin G (IgG) subclass, degree and type of glycosylation, injection route, and injection site, that could affect the pharmacokinetics (PK) of these large macromolecular therapeutics, which in turn could have ramifications on their efficacy and safety. This minireview examines how characteristics of the antibodies could be altered to change their PK profiles. For example, it was observed that a net charge modification of at least a 1-unit shift in isoelectric point altered antibody clearance. Antibodies with enhanced affinity for FcRn at pH 6.0 display longer serum half-lives and slower clearances than wild type. Antibody fragments have different clearance rates and tissue distribution profiles than full length antibodies. Fc glycosylation is perceived to have a minimal effect on PK while that of terminal high mannose remains unclear. More investigation is warranted to determine if injection route and/or site impacts PK. Nonetheless, a better understanding of the effects of all these variations may allow for the better design of antibody therapeutics.



Similar content being viewed by others
Abbreviations
- Cyno:
-
Cynomolgus monkey
- Fab:
-
Antigen binding fragment
- Fc:
-
Fragment crystallizable
- FcγR:
-
Fc gamma receptor
- FcRn:
-
Neonatal Fc receptor
- GlcNAc:
-
N-acetylglucosamine
- HC:
-
Heavy chain
- IgG:
-
Immunoglobulin G
- LC:
-
Light chain
- pI :
-
Isoelectric point
- PK:
-
Pharmacokinetics
References
Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Curr Opin Drug Discov Dev. 2007;10(1):84–96.
Sarmay G, Lund J, Rozsnyay Z, Gergely J, Jefferis R. Mapping and comparison of the interaction sites on the Fc region of IgG responsible for triggering antibody dependent cellular cytotoxicity (ADCC) through different types of human Fc gamma receptor. Mol Immunol. 1992;29(5):633–9.
Correia IR. Stability of IgG isotypes in serum. mAbs. 2010;2(3):221–32.
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50(2):131–42.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11(1–2):81–8.
Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182(12):7663–71.
Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, et al. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos Biol Fate Chem. 2010;38(4):600–5.
Khawli LA, Biela B, Hu P, Epstein AL. Comparison of recombinant derivatives of chimeric TNT-3 antibody for the radioimaging of solid tumors. Hybridoma Hybridomics. 2003;22(1):1–9.
Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67(1):254–61.
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21(12):2153–63.
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. mAbs. 2010;2(6):613–24.
Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989;143(8):2595–601.
Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol. 2005;122:117–27.
Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology. 2011;21(7):949–59.
Barrett JS, Wagner JG, Fisher SJ, Wahl RL. Effect of intraperitoneal injection volume and antibody protein dose on the pharmacokinetics of intraperitoneally administered IgG2a kappa murine monoclonal antibody in the rat. Cancer Res. 1991;51(13):3434–44.
Xu Z, Wang Q, Zhuang Y, Frederick B, Yan H, Bouman-Thio E, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010;50(3):276–84.
Ternant D, Paintaud G. Pharmacokinetics and concentration–effect relationships of therapeutic monoclonal antibodies and fusion proteins. Expert Opin Biol Ther. 2005;5 Suppl 1:S37–47.
Andya JD, Hsu CC, Shire SJ. Mechanisms of aggregate formation and carbohydrate excipient stabilization of lyophilized humanized monoclonal antibody formulations. AAPS pharmSci. 2003;5(2):E10.
Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–92.
Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43.
Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol Sci. 2008;29(2):57–61.
Lee HJ, Pardridge WM. Monoclonal antibody radiopharmaceuticals: cationization, pegylation, radiometal chelation, pharmacokinetics, and tumor imaging. Bioconjug Chem. 2003;14(3):546–53.
Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10(3):455–72.
Kobayashi H, Le N, Kim IS, Kim MK, Pie JE, Drumm D, et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 1999;59(2):422–30.
Khawli LA, Glasky MS, Alauddin MM, Epstein AL. Improved tumor localization and radioimaging with chemically modified monoclonal antibodies. Cancer Biother Radiopharm. 1996;11(3):203–15.
Zheng Y, Tesar DB, Benincosa L, Birnböck H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. mAbs. 2012;4(2):0–1.
Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, et al. Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel PEDS. 2010;23(5):385–92.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25.
Zheng Y, Scheerens H, Davis Jr JC, Deng R, Fischer SK, Woods C, et al. Translational pharmacokinetics and pharmacodynamics of an FcRn-variant anti-CD4 monoclonal antibody from preclinical model to phase I study. Clin Pharmacol Ther. 2011;89(2):283–90.
Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–3.
Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17(1):104–18.
Deng R, Jin F, Prabhu S, Iyer S. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin Drug Metab Toxicol. 2012;8(2):141–60.
Millward TA, Heitzmann M, Bill K, Langle U, Schumacher P, Forrer K. Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biol J Int Assoc Biol Stand. 2008;36(1):41–7.
Wright A, Morrison SL. Effect of C2-associated carbohydrate structure on Ig effector function: studies with chimeric mouse-human IgG1 antibodies in glycosylation mutants of Chinese hamster ovary cells. J Immunol. 1998;160(7):3393–402.
Wright A, Sato Y, Okada T, Chang K, Endo T, Morrison S. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology. 2000;10(12):1347–55.
Sweetser MT, Woodworth J, Swan S, Ticho B. Results of a randomized open-label crossover study of the bioequivalence of subcutaneous versus intramuscular administration of alefacept. Dermatol Online J. 2006;12(3):1.
Xu ZH, Wang QM, Zhuang YL, Frederick B, Yan H, Marini JC, et al. Bioavailability of golimumab, an anti-tumor necrosis factor-α human monoclonal antibody, administered subcutaneously at three different injection sites in healthy subjects. Clin Pharmacol Ther. 2009;85(S1):S24.
McDonald TA, Zepeda ML, Tomlinson MJ, Bee WH, Ivens IA. Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther. 2010;12(4):461–70.
ACKNOWLEDGMENTS
The authors would like to thank Frank-Peter Theil, Enzo Palma, Wendy Putnam, and Devin Tesar for helpful discussion.
Conflict of Interest Statement
All authors are employees of Genentech, Inc., a member of the Roche Group, and are Roche stockholders.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Craig Svensson, Joseph Balthasar, and Frank-Peter Theil
Rights and permissions
About this article
Cite this article
Bumbaca, D., Boswell, C.A., Fielder, P.J. et al. Physiochemical and Biochemical Factors Influencing the Pharmacokinetics of Antibody Therapeutics. AAPS J 14, 554–558 (2012). https://doi.org/10.1208/s12248-012-9369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9369-y