Abstract
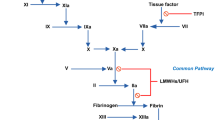
Patients undergoing anticoagulation with heparin or low molecular weight heparin (LMWH) require a superior antidote that possesses more selective biological actions and a better safety profile than protamine. We had previously developed 2 low molecular weight protamine (LMWP) fractions (TDSP4 and TDSP5) from thermolysin-digested protamine as potential nontoxie, heparin-neutralizing agents. In this, the second article in this series, studies focused on in vitro evaluation of heparin/LMWH-neutralizing efficacy and putative toxicity. These LMWP fractions, particularly TDSP5, were effective and fully capable of neutralizing a broad spectrum of heparin-induced anticoagulant activities (ie, aPTT, anti-Xa, and anti-IIa activities). Additionally, these LMWP fractions could neutralize the activities of commercial LMWH. As assessed by the anti-Xa assay, TDSP5 was as effective as, although less potent than, protamine in reversing the activity of Mono-Embolex (molecular weight 5000–7000) and 2 other different sizes (molecular weight of 3000 and 5000 d) of LMWH preparations. Furthermore, compared with protamine, TDSP5 exhibited a much-reduced toxicity and thus an improved safety profile, as reflected by its reduced ability to activate the complement system and cross-react with the antiprotamine antibodies, which are 2 primary indices of protamine toxicity.
Similar content being viewed by others
References
Jones GR, Hashim R, Power DM. A comparison of the strength of binding of antithrombin III, protamine and poly(1-lysine) to heparin samples of different anticoagulant activities. Biochim Biophys Acta. 1986;883:69–76.
Metz S, Horrow JC. Protamine and newer heparin antagonists. In: Stoelting RK. ed. Pharmacology & Physiology in Anesthetic Practice. JB Lippincott Co. Philadelphia, PA. 1994:1–15.
Porsche R, Brenner ZR. Allergy to protamine sulfate. Heart Lung. 1999;28:418–428.
Weiler JM, Gellhaus MA, Carter JG, et al. A prospective study of the risk of an immediate adverse reaction to protamine sulfate during cardiopulmonary bypass surgery. J Allergy Clin Immunol. 1990;85:713–719.
Levy JH, Zaidan JR, Faraj B. Prospective evaluation of risk of protamine reactions in patients with NPH insulin-dependent diabetes. Anesth Analg. 1986;65:739–742.
Morel DR, Lowenstein E, Nguyenduy T, et al. Acute pulmonary vasoconstriction and thromboxane release during protamine reversal of heparin anticoagulation in awake sheep: Evidence for the role of reactive oxygen metabolites following nonimmunological complement activation. Circ Res. 1988;62:905–915.
Sela M. Antigenicity: Some molecular aspects. Science. 1969;166:1365–1374.
Yang VC, Port FK, Kim JS, Teng CL, Till GO, Wakefield TW. The use of immobilized protamine in removing heparin and preventing protamine-induced complications during extracorporeal blood circulation. Anesthesiology 1991;75:288–297.
Chang LC, Lee HF, Yang Z, Yang VC. Low molecular weight protamine as nontoxic heparin low molecular weight heparin antidote (I): Preparation and characterization. AAPS PhamSci. 2001: 3 (2) article 17 (http://www.pharmsci.org/scientificjournals/pharmsci/journal/01_17.html)
Byun Y, Singh VK, Yang VC. Low molecular weight protamine: A potential nontoxic heparin antagonist. Thromb Res. 1999;94:53–61.
Mayer MM, In: Kabat EA, Mayer MM, eds. Expermental immunochemistry (Thomas, Springfield, II., 1961:113.
Cooper HN, Paterson Y. Production of antibodies. In: Coligan JE, Kruisbbel AM, Margulies DH, eds. Current protocols in immunology (Green Publishing Associates and Wiley-Interscience. New York, NY. 1991;241–247.
Hirsh J, Dalen JE, Deykin D, Poller L. Heparin: Mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1992;102:337S-351S.
Oosta GM, Gardner WT, Beeler DL, Rosenberg RD. Multiple functional domains of the heparin molecule. Proc Natl Acad Sci U S A. 1981;78:829–833.
Casu B. Structure and biological activity of heparin. Adv Carbohydr Chem Biochem. 1985;43:51–134.
Rosenberg R. The heparin-antithrombin system: A natural anticoagulant mechanism. In: Colman RW, Marder VJ, Hirsh J, eds. Hemostasis and thrombosis: Basic principles and clinical practice. JB Lippincott, Philadelphia, PA: 1987:1373–1392.
Bernat A, Herbert JM. Protamine sulphate inhibits pentasaccharide (sr80027)-induced bleeding without affecting its antithrombotic and anti-factor Xa activity in the rat. Haemostasis. 1996;26:195–202.
Buchanan MR, Ofosu FA, Fernandez F, Van Ryn J. Lack of relationship between enhanced bleeding induced by heparin and other sulfated polysaccharides and enhanced catalysis of thrombin inhibition. Semin Thromb Hemost 1986;12:324–327.
Coccheri S. Low molecular weight heparins: An introduction. Haemostasis. 1990;20(suppl 1):74–80.
AHFS Drug Information 1996. Protamine sulfate; 2504-2505.
Kirklin JW, Barratt-Boyes BG. Cardiac surgery: Morphology, diagnostic criteria, natural history, techiques, results, and indications. Wiley, New York, NY; 1986.
Kirklin JK, Chenoweth DE, Naftel DC, et al. Effects of protamine administration after cardiopulmonary bypass on complement, blood elements, and the hemodynamic state. Ann Thorac Surg. 1986;41:193–199.
Rent R, Ertel N, Eisenstein R, Gewurz H. Complement activation by interaction of polyanions and polycations. I. Heparin-protamine induced consumption of complement. J Immunol. 1975;114:120–124.
Cavarocchi NC, Schaff HV, Orszulak TA, Homburger HA, Schnell WA Jr, Pluth JR. Evidence for complement activation by protamine-heparin interaction after cardiopulmonary bypass. Surgery. 1985;98:525–531.
Nell LJ, Thomas JW. Frequency and specificity of protamine antibodies in diabetic and control subjects. Diabetes. 1988;37:172–176.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: July 11, 2001
Rights and permissions
About this article
Cite this article
Chang, LC., Liang, J.F., Lee, HF. et al. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (II): In vitro evaluation of efficacy and toxicity. AAPS PharmSci 3, 18 (2001). https://doi.org/10.1208/ps030318
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps030318