Abstract
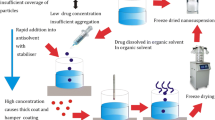
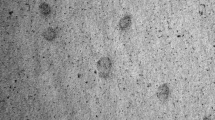
Present study was aimed to increase the oral bioavailability and reduce the fast fed variability of Ibrutinib by developing nanosuspension by simple precipitation-ultrasonication method. A three factor, three level, box-behnken design was used for formulation optimization using pluronic F-127 as stabilizer. Size and polydispersity index of the developed formulations were in the range of 278.6 to 453.2 nm and 0.055 to 0.198, respectively. Field emission scanning electron microscope (FESEM) revealed discrete units of nanoparticles. Further, differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) studies confirmed the transformation of crystal drug to amorphous. The amorphous nature was retained after 6-month storage at room temperature. Size reduction to nano range and polymorphic transformation (crystalline to amorphous) increased the solubility of nanosuspension (21.44-fold higher as compared to plain drug). In vivo studies of plain drug suspension displayed a significant pharmacokinetic variation between fasting and fed conditions. The formulation had shown increased Cmax (3.21- and 3.53-fold), AUC0-t (5.21- and 5.83-fold) in fasting and fed states compared to that of values obtained for plain drug in fasting state (Cmax 48.59 ± 3.30 ng/mL and AUC0-t 137.20 ± 35.47 ng.h/mL). Significant difference was not observed in the pharmacokinetics of nanosuspension in fasting and fed states. The formulation had improved solubility in the intestinal pH, which might be the driving force behind the decreased precipitation and increased absorption at intestinal region. Optimistic results demonstrated nanosuspension as a promising approach for increasing the solubility, extent of absorption and diminishing the fast fed variability.











Similar content being viewed by others
References
Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma. 2013;54(11):2385–91.
Kokhaei P, Jadidi-Niaragh F, Sotoodeh Jahromi A, Osterborg A, Mellstedt H, Hojjat-Farsangi M. Ibrutinib-a double-edge sword in cancer and autoimmune disorders. J Drug Target. 2016;24(5):373–85.
Smith MR. Ibrutinib in B lymphoid malignancies. Expert Opin Pharmacother. 2015;16(12):1879–87.
Massó-Vallés D, Jauset T, Soucek L. Ibrutinib repurposing: from B-cell malignancies to solid tumors. Oncoscience. 2016;3(5–6):147.
Haura EB, Rix U. Deploying ibrutinib to lung cancer: another step in the quest towards drug repurposing. J Natl Cancer Inst. 2014;106(9).
EMA. CHMP assessment report: European Medicines Agency; 2014. https://www.ema.europa.eu/en/documents/assessment-report/imbruvica-epar-public-assessment-report_en.pdf.
Shakeel F, Iqbal M, Ezzeldin E. Bioavailability enhancement and pharmacokinetic profile of an anticancer drug ibrutinib by self-nanoemulsifying drug delivery system. J Pharm Pharmacol. 2016;68(6):772–80.
De Jong J, Sukbuntherng J, Skee D, Murphy J, O’Brien S, Byrd JC, et al. The effect of food on the pharmacokinetics of oral ibrutinib in healthy participants and patients with chronic lymphocytic leukemia. Cancer Chemother Pharmacol. 2015;75(5):907–16.
Shono Y, Jantratid E, Kesisoglou F, Reppas C, Dressman JB. Forecasting in vivo oral absorption and food effect of micronized and nanosized aprepitant formulations in humans. Eur J Pharm Biopharm. 2010;76(1):95–104.
Qiu Q, Lu M, Li C, Luo X, Liu X, Hu L, et al. Novel self-assembled Ibrutinib-phospholipid complex for potently Peroral delivery of poorly soluble drugs with pH-dependent solubility. AAPS PharmSciTech. 2018;19(8):3571–83.
Kesisoglou F, Mitra A. Crystalline nanosuspensions as potential toxicology and clinical oral formulations for BCS II/IV compounds. AAPS J. 2012;14(4):677–87.
He J, Han Y, Xu G, Yin L, Neubi MN, Zhou J, et al. Preparation and evaluation of celecoxib nanosuspensions for bioavailability enhancement. RSC Adv. 2017;7(22):13053–64.
Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19.
Guo L, Kang L, Liu X, Lin X, Di D, Wu Y, et al. A novel nanosuspension of andrographolide: preparation, characterization and passive liver target evaluation in rats. Eur J Pharm Sci. 2017;104:13–22.
Park J, Sun B, Yeo Y. Albumin-coated nanocrystals for carrier-free delivery of paclitaxel. J Control Release. 2017;263:90–101.
Danhier F, Ucakar B, Vanderhaegen M-L, Brewster ME, Arien T, Préat V. Nanosuspension for the delivery of a poorly soluble anti-cancer kinase inhibitor. Eur J Pharm Biopharm. 2014;88(1):252–60.
Sahu BP, Das MK. Nanosuspension for enhancement of oral bioavailability of felodipine. Appl Nanosci. 2014;4(2):189–97.
Reverchon E. Supercritical antisolvent precipitation of micro-and nano-particles. J Supercrit Fluids. 1999;15(1):1–21.
Pathak P, Meziani MJ, Desai T, Sun Y-P. Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing. J Supercrit Fluids. 2006;37(3):279–86.
Sarkari M, Brown J, Chen X, Swinnea S, Williams RO III, Johnston KP. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int J Pharm. 2002;243(1–2):17–31.
Chen X, Young TJ, Sarkari M, Williams RO III, Johnston KP. Preparation of cyclosporine a nanoparticles by evaporative precipitation into aqueous solution. Int J Pharm. 2002;242(1–2):3–14.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Gera S, Talluri S, Rangaraj N, Sampathi S. Formulation and evaluation of Naringenin Nanosuspensions for bioavailability enhancement. AAPS PharmSciTech. 2017;18(8):3151–62.
Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, et al. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495(2):738–49.
Matteucci ME, Hotze MA, Johnston KP, Williams RO. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006;22(21):8951–9.
Singh MK, Pooja D, Ravuri HG, Gunukula A, Kulhari H, Sistla R. Fabrication of surfactant-stabilized nanosuspension of naringenin to surpass its poor physiochemical properties and low oral bioavailability. Phytomedicine. 2018;40:48–54.
Rahim H, Sadiq A, Khan S, Khan MA, Shah SMH, Hussain Z, et al. Aceclofenac nanocrystals with enhanced in vitro, in vivo performance: formulation optimization, characterization, analgesic and acute toxicity studies. Drug Des Devel Ther. 2017;11:2443–52.
Wang L, Du J, Zhou Y, Wang Y. Safety of nanosuspensions in drug delivery. Nanomedicine. 2017;13(2):455–69.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1–2):216–22.
Kassem MA, ElMeshad AN, Fares AR. Enhanced solubility and dissolution rate of lacidipine nanosuspension: formulation via antisolvent sonoprecipitation technique and optimization using box–Behnken design. AAPS PharmSciTech. 2017;18(4):983–96.
Mishra V, Kesharwani P, Amin MC, Iyer A, editors. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. Academic Press; 2017 May 23
Kaithwas V, Dora CP, Kushwah V, Jain S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf B: Biointerfaces. 2017;154:10–20.
Shah P, Mashru R, Rane Y, Badhan A. Design and optimization of artemether microparticles for bitter taste masking. Acta Pharma. 2008;58(4):379–92.
Kan S, Lu J, Liu J, Wang J, Zhao Y. A quality by design (QbD) case study on enteric-coated pellets: screening of critical variables and establishment of design space at laboratory scale. AJPS. 2014;9(5):268–78.
Shi X, Song S, Ding Z, Fan B, Huang W, Xu T. Improving the solubility, dissolution, and bioavailability of Ibrutinib by preparing it in a Coamorphous state with saccharin. J Pharm Sci. 2019;108:3020–8.
Baumgartner R, Teubl BJ, Tetyczka C, Roblegg E. Rational design and characterization of a nanosuspension for intraoral administration considering physiological conditions. J Pharm Sci. 2016;105(1):257–67.
Shariare MH, Sharmin S, Jahan I, Reza H, Mohsin K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J Drug Deliv Sci Technol. 2018;43:122–8.
Liu D, Pan H, He F, Wang X, Li J, Yang X, et al. Effect of particle size on oral absorption of carvedilol nanosuspensions: in vitro and in vivo evaluation. Int J Nanomedicine. 2015;10:6425.
Colombo M, Staufenbiel S, Rühl E, Bodmeier R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int J Pharm. 2017;521(1–2):156–66.
Zhang X, Guan J, Ni R, Li LC, Mao S. Preparation and solidification of redispersible nanosuspensions. J Pharm Sci. 2014;103(7):2166–76.
Kho K, Cheow WS, Lie RH, Hadinoto K. Aqueous re-dispersibility of spray-dried antibiotic-loaded polycaprolactone nanoparticle aggregates for inhaled anti-biofilm therapy. Powder Technol. 2010;203(3):432–9.
Afifi SA, Hassan MA, Abdelhameed AS, Elkhodairy KA. Nanosuspension: an emerging trend for bioavailability enhancement of etodolac. Int J Polym Sci. 2015;2015:1–16.
Karakucuk A, Celebi N, Teksin ZS. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. a Design of Experiment approach. Eur J Pharm Sci. 2016;95:111–21.
Doktorovová S, Araújo J, Garcia ML, Rakovský E, Souto EB. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf B: Biointerfaces. 2010;75(2):538–42.
Gadadare R, Mandpe L, Pokharkar V. Ultra rapidly dissolving repaglinide nanosized crystals prepared via bottom-up and top-down approach: influence of food on pharmacokinetics behavior. AAPS PharmSciTech. 2015;16(4):787–99.
Talekar M, Ganta S, Amiji M, Jamieson S, Kendall J, Denny WA, et al. Development of PIK-75 nanosuspension formulation with enhanced delivery efficiency and cytotoxicity for targeted anti-cancer therapy. Int J Pharm. 2013;450(1–2):278–89.
Singh A, Neupane YR, Panda BP, Kohli K. Lipid based nanoformulation of lycopene improves oral delivery: formulation optimization, ex vivo assessment and its efficacy against breast cancer. J Microencapsul. 2017;34(4):416–29.
Berben P, Ashworth L, Beato S, Bevernage J, Bruel J-L, Butler J, et al. Biorelevant dissolution testing of a weak base: Interlaboratory reproducibility and investigation of parameters controlling in vitro precipitation. Eur J Pharm Biopharm. 2019;140:141–8.
Dai W-G, Dong LC, Li S, Deng Z. Combination of Pluronic/vitamin E TPGS as a potential inhibitor of drug precipitation. Int J Pharm. 2008;355(1–2):31–7.
Dalvi SV, Dave RN. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res. 2009;48(16):7581–93.
Kakran M, Sahoo NG, Tan I-L, Li L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J Nanopart Res. 2012;14(3):757.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2):16.
Lee J, Lee S-J, Choi J-Y, Yoo JY, Ahn C-H. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur J Pharm Sci. 2005;24(5):441–9.
Pitto-Barry A, Barry NP. Pluronic® block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advances. Polym Chem. 2014;5(10):3291–7.
Shaarani S, Hamid SS, Kaus NHM. The influence of Pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of Thymoquinone drug. Pharm Res. 2017;9(1):12.
Chouhan P, Saini T. D-optimal design and development of microemulsion based transungual drug delivery formulation of ciclopirox olamine for treatment of onychomycosis. Indian J Pharm Sci. 2016;78(4):498–511.
Lin Y, Alexandridis P. Temperature-dependent adsorption of Pluronic F127 block copolymers onto carbon black particles dispersed in aqueous media. J Phys Chem B. 2002;106(42):10834–44.
Instruments M. Zetasizer nano series user manual. MAN0317. 2004;1:2004.
Hassani S, Laouini A, Fessi H, Charcosset C. Preparation of chitosan–TPP nanoparticles using microengineered membranes–effect of parameters and encapsulation of tacrine. Colloids Surf A Physicochem Eng Asp. 2015;482:34–43.
Shah B, Khunt D, Bhatt H, Misra M, Padh H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parameters. Eur J Pharm Sci. 2015;78:54–66.
Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295.
Chen Z, Zhai J, Liu X, Mao S, Zhang L, Rohani S, et al. Solubility measurement and correlation of the form a of ibrutinib in organic solvents from 278.15 to 323.15 K. J Chem Thermodyn. 2016;103:342–8.
Eloy JO, Marchetti JM. Solid dispersions containing ursolic acid in Poloxamer 407 and PEG 6000: a comparative study of fusion and solvent methods. Powder Technol. 2014;253:98–106.
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, et al. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using box-Behnken design. AAPS PharmSciTech. 2015;16(1):118–28.
Karolewicz B, Gajda M, Górniak A, Owczarek A, Mucha I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J Therm Anal Calorim. 2017;130(1):383–90.
Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: I. preparation by a size-reduction technique. Int J Pharm. 1998;160(2):229–37.
Liu D, Xu H, Tian B, Yuan K, Pan H, Ma S, et al. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation–ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech. 2012;13(1):295–304.
Nagaraj K, Narendar D, Kishan V. Development of olmesartan medoxomil optimized nanosuspension using the box–Behnken design to improve oral bioavailability. Drug Dev Ind Pharm. 2017;43(7):1186–96.
Beg S, Chaudhary V, Sharma G, Garg B, Panda SS, Singh B. QbD-oriented development and validation of a bioanalytical method for nevirapine with enhanced liquid–liquid extraction and chromatographic separation. Biomed Chromatogr. 2016;30(6):818–28.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30(2):307–24.
Acknowledgements
The authors would like to thank NIPER-HYD and Department of Pharmaceuticals (DoP) for providing the facilities and for funding the research work.
Funding
The NIPER-HYD and Department of Pharmaceuticals (DoP) provided funding for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rangaraj, N., Pailla, S.R., Chowta, P. et al. Fabrication of Ibrutinib Nanosuspension by Quality by Design Approach: Intended for Enhanced Oral Bioavailability and Diminished Fast Fed Variability. AAPS PharmSciTech 20, 326 (2019). https://doi.org/10.1208/s12249-019-1524-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1524-7