Abstract
Background
Congenital heart disease (CHD) is a multifactorial birth defect which has variable demographic characteristics among children in different geographical areas. This study aimed to detect the distribution of demographic data, perinatal risk factors, types, age, and mode of presentation of CHD among Egyptian children.
Results
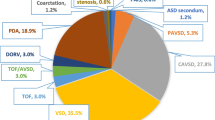
The medical records of 1005 patients were included. They were 545 males (54%) and 462 females (46%) with a ratio of 1.2:1. Acyanotic CHD was encountered in 79.2%. Isolated ventricular septal defect and tetralogy of Fallot were the most common acyanotic and cyanotic lesions, respectively. The majority was diagnosed within the first year of life (86.7%) and was born to young mothers (91.3%). The accidental discovery of a murmur was the most frequent presentation (35%). Heart failure was detected in 44%, audible murmurs in 74.4%, maternal illnesses in 54%, consanguinity in 44.6%, prematurity in 19.3%, assisted reproduction in 11.7%, family history of CHD in 9.2%, abortions in 7.1%, and extracardiac anomalies in 3.6% of the studied population. Down syndrome (DS) was the most commonly occurring chromosomal anomaly, and the atrioventricular septal defect was the most characteristic cardiac lesion found among them.
Conclusions
There is no sex predilection among Egyptian children with CHD. Most of the cases are diagnosed in early infancy. Accidental discovery of a murmur is the most common mode of presentation. A variety of predisposing risk factors are abundant in the Egyptian population. DS is the most common chromosomal anomaly linked to CHD. Establishment of a national medical birth registry containing all information about all births in Egypt is needed for adequate surveillance and monitoring of perinatal health problems and congenital birth defects so that preventive measures can be early implemented. Proper and detailed data collection should be fulfilled in the medical records of every single patient.
Similar content being viewed by others
Highlights
-
The majority of patients (79.2%) had acyanotic CHD.
-
VSD is the most commonly encountered acyanotic CHD.
-
Fallot’s tetralogy is the most commonly occurring cyanotic CHD.
-
No sex predilection among the studied population.
-
Consanguinity is prevalent in 44.6% of the studied population.
Background
Congenital heart disease (CHD) is one of the relatively common congenital disabilities whose prevalence ranges from 3.5-17.5 per 1000 live births [1]. They are becoming an increasing cause of pediatric mortality [2], especially in the developing countries [3]. The clinical spectrum of CHD is versatile and changes according to the age of presentation. Asymptomatic presentation is common and discovered accidentally on routine checkup visits, whereas other presentations can range from poor suckling, cyanosis, and shortness of breath up to frank heart failure [4].
Most of these defects follow the multifactorial pattern of inheritance as a result of interlinking genetic and environmental factors with a smaller percentage being linked to chromosomal aberrations [5]. The pattern of risk factors for CHD is different among different parts of the world. In developing countries, consanguinity is relatively prevalent; most mothers are housewives, non-smokers, and non-alcohol consumers [6]. Unfortunately, only a few studies had evaluated the perinatal risk factors among those populations [7].
A meticulous study of the epidemiology of CHDs is the cornerstone for better identification of the etiology for cardiac dysmorphogenesis so that every opportunity can be offered to prevent them prenatally [8,9,10]. Unfortunately, the epidemiology of CHD had not been thoroughly studied among Egyptian children; hence, this study aimed to review the risk factor portfolio, relative frequencies of each type of CHD, demographic characteristics, age, and mode of clinical presentations among Egyptian children with CHD; so that appropriate changes in preventive health policies can be implemented and optimum care for such patients can be provided.
Methods
This is a retrospective epidemiological cross-sectional study that included all children with confirmed CHDs who are registered in the Pediatric Cardiology Clinic at a Children’s University Hospital which is considered a tertiary referral center that provides medical services for a large geographical sector in Cairo Governorate (mainly urban areas). Collaboration between all departments (pediatrics, cardiology, fetal ultrasound unit, and cardiothoracic surgery) takes place in this university hospital to provide all the necessary facilities needed for proper diagnosis and management of children with CHD (including advanced pediatric echocardiography, cardiac catheterization, fetal echocardiography, and cardiothoracic surgeries).
The study was conducted over a period of 3 years from January 2013 till December 2015. It included all children with confirmed structural CHD who were diagnosed from the first day of life up till the age of 12 years. Patients with congenital cardiomyopathies and congenital heart block were excluded from the study.
The files of 1005 patients were revised in detail. Some missing data were detected in 57 files and were completed by telephone calls when a valid number was available. Diagnosis of the type of CHDs was confirmed by echocardiography and documented in the files. Data collected included full demographic and clinical data. A thorough study of the perinatal (antenatal, natal, and postnatal), as well as the family history, was done to detect possible underlying risk factors for CHDs.
Demographic data included age at diagnosis, gender, sex, consanguinity, parental education, and occupation. For each studied case, the presenting complaint, type of CHD, presence of dysmorphic features, associated syndromes, or other congenital anomalies were all reported. Complete risk factor assessment was done for each reviewed file. Antenatal history included maternal age at conception, full obstetric history, repeated abortions or stillbirths, type of conception (normal or assisted), medical diseases with pregnancy (i.e., diabetes mellitus, hypertension, systemic lupus, and bronchial asthma), teratogen exposure (radiation, chemicals and smoking), maternal infection (i.e., urinary tract infection, congenital TORCH infections, and premature rupture of membranes), maternal medications during pregnancy (i.e., hypoglycemic, antihypertensive, antiepileptic, antibiotics). Natal and postnatal history included the gestational age (GA), early neonatal illnesses, and NICU admissions. Family history included the presence of CHDs, other congenital or chromosomal abnormalities, and sibling deaths.
Statistical methods
Data were collected, tabulated, and analyzed using SPSS, version 12. Mean and standard deviation were used for quantitative variables. Frequency and percentages were used for qualitative variables.
Results
Over the 3 years study period, a total number of 1005 of patients were included. They were 543 males (54%) and 462 females (46 %), their ages at diagnosis ranged from 1 day to 12 years with a median and interquartile range (IQR) of 6 (9-0.5) months. Of the studied population, acyanotic CHD was encountered in 796 (79.2%) whereas cyanotic CHD in 209 (20.8%). The isolated ventricular septal defect was the most common acyanotic CHD (19.8%), while tetralogy of Fallot (9.8%) was the most common cyanotic CHD. Small hemodynamically insignificant patent foramen ovale (PFO) and patent ductus arterisus (PDAs) were considered normal. Most of our patients had been diagnosed within the first year of life (48.9% in the early infancy and 37.8% in the neonatal period). The relative frequencies and ages of presentations of individual types of CHDs are shown in Table 1.
The accidental discovery of a murmur was the most common presenting complaint (Fig. 1). Audible murmurs were detected in 748 (74.4%) and heart failure in 442 (44%) of patients. Hospital admissions were encountered in 469 patients; of them, 270 were admitted due to recurrent chest infections, 91 for cyanotic spells, 75 for failure to thrive, and 33 for neonatal sepsis-like illnesses. Life-saving cardiac interventions were performed in the first year of life for 33 patients; modified Blalock-Tausing shunt for 24 patients with tetralogy of Fallot and balloon atrial septostomy for 9 patients with TGA. Most of the patients (91.3%) were born to young mothers aged < 29 years, and (66.3%) were born to multiparous mothers. Down syndrome was the most common chromosomal anomaly (10.4%) found and atrioventricular septal (AVSD) defect was the most frequently occurring cardiac lesion among them (40.3%). Fetal echocardiography was performed for 47 patients with assisted reproduction; of them, 18 patients had shown abnormal cardiac scanning. Distribution of demographic data and risk factors is shown in Table 2.
Discussion
Clinical presentation of CHD is versatile and is age-dependent, and hence, a higher index of suspicion is needed for early diagnosis and treatment [11]. In this study, the commonest presentation for CHD was the accidental discovery (35%) followed by recurrent chest infections (30.2%), cyanosis (16.7%), failure to thrive (13%), neonatal sepsis-like illness (3.3%), and finally shortness of breath (1.7%). In a study done by Otaigbe and Tabansi [3], indications for screening echocardiography were auscultation of a murmur (36%), rapid breathing (19.8%), failure to gain weight (11%), and cyanosis (9.9%), whereas in a study done by George and Frank-Briggs [11], fast breathing and inability to gain weight were the commonest presenting symptoms among CHD children.
Prevalence of murmurs is variable in different studies as it depends on the clinical skills, frequency, and timing of examination as nearly half of newborns with CHD will have no murmurs and possibly no other signs when examined at birth [12]. In this study, we detected audible murmurs in 74.4% of patients. Nearly 2 to 3 out of 1000 neonates with CHD will reveal symptoms during their first year of life. Diagnosis will be made by the first week in 40-50% and by the end of the first month in 50-60% of them [13]. In this study, most of our patients (86.8%) were diagnosed below the age of 1 year (37.8% in the neonatal period, and 49% within the first year of life). Similarly, in a study done by Subramanyan et al. [14], the ages at diagnosis of their CHD cases were in the early infancy and the neonatal periods in 40% and 38% of their studied population respectively.
In this study, we detected a male to female ratio of 1.2:1. This matches with previous studies [3, 15]. Isolated VSD was the most prevalent acyanotic CHD. This matches with most of the previous studies [3, 8, 15], whereas tetralogy of Fallot was the most frequent cyanotic CHD, which is also in agreement with most of the available studies [16,17,18].
CHD is the most common etiology for the occurrence of heart failure in infancy [19]. In the present study, we detected heart failure in 44% of the studied population. Similarly, Sommers et al. [20] detected heart failure in 39.1% of patients with CHD. The relatively higher prevalence of complications encountered in this study could be attributed to the lack of regular follow up and non-compliance to treatment, which eventually lead to the delay in surgical management.
About 15% of CHD cases could be related to an underlying genetic cause, and a smaller percentage could be attributed to an environmental modifiable risk factor [21]. According to Liu et al. [22], nearly 14% of CHD cases could be prevented by avoidance of exposure to all known risk factors; however, some recent studies detected a higher percentage of about 30% [21, 22]. Through this study, we tried to review the distribution of the perinatal risk factors-already known in literature among our studied population. This work was not designed to study them individually as predictors for the occurrence of CHDs; also, due to missing data in some files; not all the known risk factors were studied. This included maternal body mass index, smoking, anemia, nutritional status, and vitamin and folic acid supplementation during pregnancy.
In this study, the majority of our patients (91.7%) belonged to housewives. Different maternal occupations have been linked to CHDs [23]. Scientific research has not yet confirmed the association between maternal workplace exposure during pregnancy and the possible teratogenic effect on the forthcoming fetus, though there are still some concerns regarding exposure to pesticides, organic solvents, and heavy metals [24].
In Egypt, the prevalence of consanguinity is 29% [25]. The relation between consanguinity and the incidence of CHD had been explored in previous studies [26, 27]. We detected consanguinity and positive family history of CHD in 44.6% and 9.2% of our studied population, respectively. Similarly, Hag et al. [28] detected consanguinity and positive family history in 49% and 14% of their studied population, respectively, whereas Fung et al. [29] detected them in 3.5% and 21.8% respectively and detected 9% prevalence of CHD among first-degree relatives. Also, Nabulsi et al. [5] and AL–Ani [30] detected consanguinity rates of 34.7% and 77.9%, respectively. The differences among various studies reflect the differences in the prevalence of consanguinity among different societies. Moreover, the high-risk factor in closely related parents indicates that consanguinity may act as a genetic predisposition that increases the susceptibility of developing CHD, especially when there is exposure to an environmental risk factor. This highlights the need for public health education regarding the hazards of inbreeding.
In this study, one hundred and eighteen patients (11.7% of our studied population) were the product of assisted reproduction. Children conceived via modern technologies are thought to be at a higher risk for developing birth defects, including CHD [31]. Koivurova et al. [32] detected a fourfold increase in the incidence of CHD among fetuses conceived via in vitro fertilization (IVF). Also, Tararbit et al. [33] found a 40% increase in the risk of CHD among those children.
Chromosomal anomalies account for (8–10%) of syndromatic CHDs [34] with Down syndrome (DS) being the most common chromosomal anomaly seen among them [35]. In this study, syndromatic CHD was present in 16.7% of the studied population (62.5% of them were DS). Fung et al. [29] detected genetic and syndromatic CHD in 9.5% of their studied population. There is geographic variability in the type of the dominant cardiac lesion seen in DS among different countries [36]. In this study, the atrioventricular septal defect was the commonest lesion seen among DS (40.3%). This matches with Benhaourech et al. [37].
The pattern of maternal age as a hazardous factor for congenital defects differs among different countries which imply possible underlying genetic and environmental background rather than only the biological age [38]. Some studies suggested that the gynecological immaturity [39], lack of proper antenatal care, low socioeconomic class, poor diet, and other environmental non-biological factors account for birth defects among young mothers [40]. Other studies had observed the prevalence of CHDs among older mothers [41, 42], whereas Best and Rankin [43] failed to find a strong evidence to support that advanced maternal age is a risk factor for CHD. Older maternal age has been linked to chromosomal-related congenital abnormalities while the risk of maternal age on the non-chromosomal abnormalities is considered negligible [38]. In this study, most of our patients (91.3%) belonged to young mothers (< 29 years old), only 5.9% belonged to mothers older than 35 years at conception, and only 16.7% were associated with syndromatic and chromosomal abnormalities.
Various studies had shown the effect of maternal diabetes as a risk factor for fetal cardiac malformations [44], as well as maternal hypertension, cigarette smoking, and other maternal chronic illnesses [22]. In this study, maternal diabetes, asthma, hypertension, and epilepsy were found in 27.9%, 14.5%, 7.4%, and 4.2% of the studied population, respectively.
Though the exact etiological factors that link the association between increasing maternal parity and the risk of CHD is still unclear, theories include nutrient depletion especially folic acid [45], short inter-pregnancy periods [46], intrauterine exposure to teratogenic viruses (such as rubella) from children sharing the same home environment [47], biological changes in the intrauterine environment and psychological stress in pregnant mothers who are taking care of many children [48]. In this study, most of the patients (66.3%) were born to multiparous mothers, while 33.7% were born to nulliparous mothers. This matches with the meta-analysis done by Yu et al. [49].
In this study, abortions occurred in 7.1% of the studied population. In contrast to Li et al. [50] who failed to find an association between bad obstetric history, recurrent abortions, and the risk of CHD, Abqari et al. [6] detected such an association. Also, Feng et al. [51] found that mothers will have a 24% higher risk of cardiac anomalies in their children if they experienced repeated abortions before. Etiological arguments include possible uterine factors that influence the implanted embryo [52] and associated chronic maternal illnesses [53]. In this study, we also detected prematurity in 19.3% of our studied patients. Tanner et al. [54] found that preterm infants are 2-times prone to CHD when compared to term infants; they detected prematurity in 16% of their CHD patients.
Study limitations
This work was not designed to study the risk factors as predictors for the occurrence of CHD; instead, it aimed at detecting the frequency of occurrence of those risk factors already documented in literature—among our studied population. Moreover, due to missing data in files, not all the known risk factors were studied, such as maternal body mass index, anemia, nutritional status, antenatal vitamin, and folic acid supplementation. Furthermore, the exact antenatal timing of exposure to teratogen was also missing. In addition, many patients had more than one underlying possible risk factor.
Multi-centric similar studies are needed to be done in different governorates, different geographical areas, Upper and Lower Egypt, rural and urban areas, though we still believe that this study can be considered as a nidus for such studies as it was done in a large University tertiary referral center that receives patients from different geographical areas in Cairo Governorate including those critical, severe, and complicated cases that are neither managed in the Ministry of Health hospitals nor in the private sector.
Conclusions
This study represents a descriptive epidemiological review of the pattern, clinical spectrum, age of presentation, sex distribution, and risk factor portfolio of CHD among the Egyptian ethnicity. Establishment of a national medical birth registry containing all information about all births in Egypt is needed for adequate surveillance and monitoring of perinatal health problems and congenital birth defects so that preventive measures can be early implemented through interdepartmental collaborations between different medical specialities including obstetricians, pediatricians, and geneticists. Proper and detailed data collection should be fulfilled in the medical records of every single patient.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ASD:
-
Atrial septal defect
- AVSD:
-
Atrioventricular septal defect
- CHD:
-
Congenital heart disease
- DS:
-
Down syndrome
- HLH:
-
Hypoplastic left heart
- PDA:
-
Patent ductus arteriosus
- PS:
-
Pulmonary stenosis
- TAPVR:
-
Total anomalous pulmonary venous return
- TGA:
-
Transposition of great arteries
- VSD:
-
Ventricular septal defect
References
Bolisetty S, Daftary A, Ewald D et al (2004) Congenital heart defects in Central Australia. Med J Aust 180:614–617. https://doi.org/10.5694/j.1326-5377.2004.tb06122.x
Kapoor R, Gupta S (2008) Prevalence of congenital heart disease, Kanpur, India. Indian Pediatr 45:309–311
Otaigbe BE, Tabansi PN (2014) Congenital heart disease in the Niger Delta region of Nigeria: a four-year prospective echocardiographic analysis: cardiovascular topic. Cardiovasc J Afr 25:265–268. https://doi.org/10.5830/CVJA-2014-055
Wren C, Richmond S, Donaldson L (1999) Presentation of congenital heart disease in infancy: implications for routine examination. Arch Dis Child Fetal Neonatal Ed 80:F49–F53. https://doi.org/10.1136/fn.80.1.F49
Nabulsi MM, Tamim H, Sabbagh M et al (2003) Parental consanguinity and congenital heart malformations in a developing country. Am J Med Genet 116A:342–347. https://doi.org/10.1002/ajmg.a.10020
Abqari S, Gupta A, Shahab T et al (2016) Profile and risk factors for congenital heart defects: a study in a tertiary care hospital. Ann Pediatr Cardiol 9:216. https://doi.org/10.4103/0974-2069.189119
Bassili A, Mokhtar SA, Dabous NI et al (2000) Risk factors for congenital heart diseases in Alexandria, Egypt. Eur J Epidemiol 16:805–814
Alabdulgader AA (2001) Congenital heart disease in 740 subjects: epidemiological aspects. Ann Trop Paediatr 21:111–118 https://doi.org/11471253
Johar D, Ahmed SM, El Hayek S et al (2019) Diabetes-induced proteome changes throughout development. Endocr Metab Immune Disord Drug Targets 19:732–743. https://doi.org/10.2174/1871530319666190305153810
Abushouk AI, El-Husseny MWA, Bahbah EI et al (2017) Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed Pharmacother 95:692–700. https://doi.org/10.1016/j.biopha.2017.08.083
George IO, Frank-Briggs AI (2009) Pattern and clinical presentation of congenital heart diseases in Port-Harcourt. Niger J Med 18:211–214
Ainsworth SB, Wyllie JP, Wren C (1999) Prevalence and clinical significance of cardiac murmurs in neonates. Arch Dis Child - Fetal Neonatal Ed 80:F43–F45. https://doi.org/10.1136/fn.80.1.F43
Bernstein D (2004) Congenital heart disease. In: Nelson Textbook of Pediatrics, 17th edn. Canada: Hardback; Elsevier Health Sciences
Subramanyan R, Joy J, Venugopalan P et al (2000) Incidence and spectrum of congenital heart disease in Oman. Ann Trop Paediatr 20:337–341. https://doi.org/10.1080/02724936.2000.11748155
Bassili A, Mokhtar SA, Dabous NI, Zaher SR, Mokhtar MMZA (2000) Congenital heart disease among school children in Alexandria, Egypt: an overview on prevalence and relative frequencies. J Trop Pediatr 46:357–362. https://doi.org/10.1093/tropej/46.6.357
Ibadin MO, Sadoh WE, Osarogiabon W (2005) Congenital heart diseases at the University of Benin Teaching Hospital.pdf. Niger J Paediatr 32:29–32
Ramachandra N, Smitha R, Karat S et al (2006) Prevalence of congenital heart diseases in Mysore. Indian J Hum Genet 12:11. https://doi.org/10.4103/0971-6866.25296
Sani MU, Mukhtar-Yola M, Karaye KM (2007) Spectrum of congenital heart disease in a tropical environment: an echocardiography study. J Natl Med Assoc 99:665–669 https://doi.org/17595936
Massin MM, Astadicko I, Dessy H (2008) Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol 31:388–391. https://doi.org/10.1002/clc.20262
Sommers C, Nagel BHP, Neudorf U, Schmaltz AA (2005) Congestive heart failure in childhood. An epidemiologic study. Herz 30:652–662. https://doi.org/10.1007/s00059-005-2596-6
Botto LD, Lin AE, Riehle-Colarusso T et al (2007) Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol 79:714–727. https://doi.org/10.1002/bdra.20403
Liu S, Joseph KS, Lisonkova S et al (2013) Association between maternal chronic conditions and congenital heart defects. Circulation 128:583–589. https://doi.org/10.1161/CIRCULATIONAHA.112.001054
Chia S-E, Shi LM, Chan OY et al (2004) A population-based study on the association between parental occupations and some common birth defects in Singapore (1994-1998). J Occup Environ Med 46:916–923
Thulstrup AM, Bonde JP (2006) Maternal occupational exposure and risk of specific birth defects. Occup Med 56:532–543. https://doi.org/10.1093/occmed/kql115
Mokhtar MM, Abdel-Fattah MM (2001) Consanguinity and advanced maternal age as risk factors for reproductive losses in Alexandria, Egypt. Eur J Epidemiol 17:559–565
Becker SM, Al Halees Z, Molina C, Paterson RM (2001) Consanguinity and congenital heart disease in Saudi Arabia. Am J Med Genet 99:8–13
Rittler M, Liascovich R, López-Camelo J, Castilla EE (2001) Parental consanguinity in specific types of congenital anomalies. Am J Med Genet 102:36–43
Haq FU, Jalil F, Hashmi S et al (2011) Risk factors predisposing to congenital heart defects. Ann Pediatr Cardiol 4:117. https://doi.org/10.4103/0974-2069.84641
Fung A, Manlhiot C, Naik S et al (2013) Impact of prenatal risk factors on congenital heart disease in the current era. J Am Heart Assoc 2. https://doi.org/10.1161/JAHA.113.000064
Al-Ani ZR (2010) Association of consanguinity with congenital heart diseases in a teaching hospital in Western Iraq. Saudi Med J 31:1021–1027
Sutcliffe AG, Ludwig M (2007) Outcome of assisted reproduction. Lancet 370:351–359. https://doi.org/10.1016/S0140-6736(07)60456-5
Koivurova S (2002) Neonatal outcome and congenital malformations in children born after in-vitro fertilization. Hum Reprod 17:1391–1398. https://doi.org/10.1093/humrep/17.5.1391
Tararbit K, Houyel L, Bonnet D et al (2011) Risk of congenital heart defects associated with assisted reproductive technologies: a population-based evaluation. Eur Heart J 32:500–508. https://doi.org/10.1093/eurheartj/ehq440
Roos-Hesselink JW, Kerstjens-Frederikse WS, Meijboom FJ, Pieper PG (2005) Inheritance of congenital heart disease. Neth Heart J 13:88–91
Bull MJ (2011) Health supervision for children with Down syndrome. Pediatrics 128:393–406. https://doi.org/10.1542/peds.2011-1605
Narayanan DL, Yesodharan D, Kappanayil M et al (2014) Cardiac spectrum, cytogenetic analysis and thyroid profile of 418 children with Down syndrome from South India: a cross-sectional study. Indian J Pediatr 81:547–551. https://doi.org/10.1007/s12098-013-1088-6
Benhaourech S, Drighil A, El Hammiri A (2016) Congenital heart disease and Down syndrome: various aspects of a confirmed association. Cardiovasc J Afr 27:287–290. https://doi.org/10.5830/CVJA-2016-019
Loane M, Dolk H, Morris J (2009) Maternal age-specific risk of non-chromosomal anomalies. BJOG 116:1111–1119. https://doi.org/10.1111/j.1471-0528.2009.02227.x
Raatikainen K, Heiskanen N, Verkasalo PK, Heinonen S (2006) Good outcome of teenage pregnancies in high-quality maternity care. Eur J Public Health 16:157–161. https://doi.org/10.1093/eurpub/cki158
Wahn EH, Nissen E (2008) Sociodemographic background, lifestyle and psychosocial conditions of Swedish teenage mothers and their perception of health and social support during pregnancy and childbirth. Scand J Public Health 36:415–423. https://doi.org/10.1177/1403494807085315
Reefhuis J, Honein MA (2004) Maternal age and non-chromosomal birth defects, Atlanta--1968-2000: teenager or thirty-something, who is at risk? Birth Defects Res A Clin Mol Teratol 70:572–579. https://doi.org/10.1002/bdra.20065
Miller A, Riehle-Colarusso T, Siffel C et al (2011) Maternal age and prevalence of isolated congenital heart defects in an urban area of the United States. Am J Med Genet A 155A:2137–2145. https://doi.org/10.1002/ajmg.a.34130
Best KE, Rankin J (2016) Is advanced maternal age a risk factor for congenital heart disease? Birth Defects Res A Clin Mol Teratol 106:461–467. https://doi.org/10.1002/bdra.23507
Nielsen GL, Norgard B, Puho E et al (2005) Risk of specific congenital abnormalities in offspring of women with diabetes. Diabet Med 22:693–696. https://doi.org/10.1111/j.1464-5491.2005.01477.x
Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA (2000) Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343:1608–1614. https://doi.org/10.1056/NEJM200011303432204
Grisaru-Granovsky S, Gordon E-S, Haklai Z et al (2009) Effect of interpregnancy interval on adverse perinatal outcomes--a national study. Contraception 80:512–518. https://doi.org/10.1016/j.contraception.2009.06.006
Stuckey D (1956) Congenital heart defects following maternal rubella during pregnancy. Heart 18:519–522. https://doi.org/10.1136/hrt.18.4.519
Zhu JL, Olsen J, Sørensen HT et al (2013) Prenatal maternal bereavement and congenital heart defects in offspring: a registry-based study. Pediatrics 131:e1225–e1230. https://doi.org/10.1542/peds.2012-3024
Feng Y, Yu D, Chen T et al (2014) Maternal parity and the risk of congenital heart defects in offspring: a dose-response meta-analysis of epidemiological observational studies. PLoS One 9:e108944. https://doi.org/10.1371/journal.pone.0108944
Li N-N, Chen X-L, Liu Z et al (2015) Maternal abortion history and the risk of congenital heart defects. A case-control study. J Reprod Med 60:236–242 https://doi.org/26126309
Feng Y, Wang S, Zhao L et al (2015) Maternal reproductive history and the risk of congenital heart defects in offspring: a systematic review and meta-analysis. Pediatr Cardiol 36:253–263. https://doi.org/10.1007/s00246-014-1079-z
Rushton DI (1978) Simplified classification of spontaneous abortions. J Med Genet 15:1–9. https://doi.org/10.1136/jmg.15.1.1
Petrescu A, Berdan G, Hulea I et al (2007) Small cell carcinoma of the urinary bladder--a new case report. Rom J Morphol Embryol 48:309–314 https://doi.org/17914502
Tanner K, Sabrine N, Wren C (2005) Cardiovascular malformations among preterm infants. Pediatrics 116:e833–e838. https://doi.org/10.1542/peds.2005-0397
Acknowledgements
We thank the manager of the Children Hospital and the Head of Pediatric Cardiology Unit for their support.
Funding
None.
Author information
Authors and Affiliations
Contributions
Both authors conceived the idea and designed the study model. MM collected data from the files data, analyzed the data, and wrote the manuscript. YA revised the work. Both authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research protocol was approved by the Ethics committee of the Pediatric Department, Ain Shams University.
Committee Reference number: not available and not applicable as this was not a clinical trial (we did nothing to the patients for the sake of the study).
This was a retrospective study, all data were recruited from the files. No sampling, experiments, or even clinical examination were done for the patients for the sake of this research work.
Consent for publication
Written informed consents were obtained from the participants’ parents or their legal guardians in children who are under the age of 16 years.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Fahham, M.M., Ali, Y.A. Pattern of congenital heart disease among Egyptian children: a 3-year retrospective study. Egypt Heart J 73, 11 (2021). https://doi.org/10.1186/s43044-021-00133-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-021-00133-0




