Abstract
Objectives
Fibromyalgia Syndrome (FMS), is a chronic pain disorder with poorly understood pathophysiology. In recent years, repetitive transcranial magnetic stimulation (rTMS) has been recommended for pain relief in various chronic pain disorders. The objective of the present research was to study the effect of low frequency rTMS over the right dorsolateral prefrontal cortex (DLPFC) on pain status in FMS.
Methods
Ninety diagnosed cases of FMS were randomized into Sham-rTMS and Real-rTMS groups. Real rTMS (1 Hz/1200 pulses/8 trains/90% resting motor threshold) was delivered over the right DLPFC for 5 consecutive days/week for 4 weeks. Pain was assessed by subjective and objective methods along with oxidative stress markers. Patients were followed up for 6 months (post-rTMS;15 days, 3 months and 6 months).
Results
In Real-rTMS group, average pain ratings and associated symptoms showed significant improvement post rTMS. The beneficial effects of rTMS lasted up to 6 months in the follow-up phase. In Sham-rTMS group, no significant change in pain ratings was observed.
Conclusion
Right DLPFC rTMS can significantly reduce pain and associated symptoms of FMS probably through targeting spinal pain circuits and top-down pain modulation .
Trial registration: Ref No: CTRI/2013/12/004228.
Similar content being viewed by others
Introduction
Fibromyalgia affects nearly 2.10% of the world’s population [1]. The etiopathogenesis of FMS is largely unknown, although tender points all over the body suggest a peripheral pathology, but a considerable amount of data also points towards the sensitization of central pain processing pathways [2], dysfunctional pain inhibition [3] and abnormal cortical excitability [4]. Keeping in view the variable pathophysiology, the management strategies recommended for FMS include; pharmaceuticals, behavioral interventions, physical therapy, exercises along with other complementary and alternativemedicinal approaches [5]. However, no single treatment modality has been able to alleviate the full range of FMS symptoms. Hence, it is pertinent to search for effective alternative methods of therapy.
Transcranial magnetic stimulation is a non-invasive brain stimulation technique which is being used to manage psychiatric cases, several movement disorders and chronic pain conditions including fibromyalgia [6,7,8]. Recent study has shown that rTMS of the DLPFC induces changes in the activity of a network of structures involved in the integration and modulation of pain signals, including the thalamus, brainstem, insular and cingulate cortices [9]. A previous study by our group observed beneficial effects of low frequency rTMS and established the role of DLPFC modulation in chronic tension type headache [10]. However, the substrates of the analgesia and its longevity remains to be unravelled. We hypothesized that TMS of the right DLPFC using low-frequency rTMS could relieve pain and associated symptoms of fibromyalgia. The objectives (primary and secondary) of the study were to investigate the effect of rTMS on FMS pain and related symptoms and explore the mechanism of analgesia by recording the nociceptive flexion reflex (NFR); an objective marker of pain. Further, in order to understand the effect of right-DLPFC rTMS on descending pain inhibitory pathways, we assessed the diffuse noxious inhibitory controls (DNIC) system. To further substantiate the literature suggesting the role of oxidative stress markers in FMS [11, 12], we assessed the levels of thiobarbituric acid reactive substances (TBARS) and F2-isoprostane in our FMS patients before and after rTMS therapy.
Materials and methods
Ethical considerations and trial design
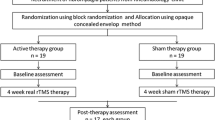
The study was conducted at the Pain Research and TMS Laboratory, Department of Physiology, All India Institute of Medical Sciences (AIIMS) New Delhi, India. Human Ethics committee of the AIIMS, New Delhi (Ref No: IESC/T-251/15.06.2013) approved the research protocol in 2013. The study was also registered in ICMR-CTRI; India (Ref No: CTRI/2013/12/004228). The study was a randomized, parallel group, placebo controlled trial. Randomization was performed through a computer generated random number table and concealment was done with sequentially numbered, sealed, opaque envelopes. Block randomization design used was to ensure equal sample size between two groups over time. The patients were blinded to the block size as well as to the treatment allocations. Participants were free to withdraw from the study at any stage. All the participants were enrolled only after obtaining the ethical approval and a duly signed informed consent form.
Study protocol
Participants were randomly assigned to either the Sham or Real rTMS group. Subjective, objective assessment of pain and estimation of oxidative stress markers were done at baseline and post-Sham/Real rTMS. Immediately post-rTMS, the patients were followed up to a period of 6 months, at the three time points; 15 days Post-rTMS, 3-months and 6-months after the therapy. During follow-up, subjective evaluation using specific questionnaires was done for a period of 6 months while NFR and DNIC were performed at post-rTMS and 15th-day post-rTMS (Fig. S1).
Sample size calculation
According to a preliminary study on fibromyalgia [13], NPRS ratings were 5·60 (1·85) at baseline which were reduced to 3·99 (1·90) after Real rTMS, while in Sham group, baseline and post treatment values were 5·34 (1.82) and 5·07 (1·89) respectively. Using this data, we required 41 cases per group to detect a significant difference between sham and real rTMS treatment in a 5% two sided t-test with 90% power. Assuming a 10% loss to the follow up, we required 45 patients of FMS per group. Accordingly a sample size of 90 patients (45 on sham treatment and 45 on real rTMS treatment) was decided.
Recruitment of FMS patients
Female patients with FMS (age, 18–50 years) having regular menstrual cycle were recruited from the Rheumatology Clinic, Department of Rheumatology, AIIMS, New Delhi; after establishing the diagnosis by a Rheumatologist. The diagnosis was based on the American College of Rheumatology criteria, 2010 for the classification of FMS [14]. Only those patients, who gave written informed consent, were enrolled for the study. The record of rescue analgesics was also maintained. The details of medication used by patients (NSAIDs, antidepressants and opioids, etc) before and after the treatment with rTMS have been given Table 1. FMS patients were excluded from the study, if they had following conditions: i) unable to give written informed consent form ii) History of seizures iii) History of seizures in first-degree relatives iv) History of any illness involving the brain v) Consumption of medications (like tramadol, acetylcholinesterase inhibitors, anticholinergics, antiemetics, antihistamines, baclofen, ß-blockers, cephalosporins, cyclosporine etc) known to lower the seizure threshold vi) History of tinnitus vii) History of bipolar disorder viii) having implants of defibrillators or neuro-stimulators or cardiac pacemakers ix) pregnant or lactating x) having chronic systemic disease, inflammatory joint diseases, secondary FM, history of trauma xi) with a concomitant diagnosis of chronic fatigue syndrome and/or any psychiatric disorder xii) currently undergoing psychotherapy.
Protocol for real/sham rTMS
MagPro R100 (MagVenture, USA) transcranial magnetic stimulator was used for repetitive magnetic stimulation of the brain. Real rTMS was administered using a butterfly coil (MCF-B70, MagVenture, USA). For selection of the optimal scalp area on the right motor cortex; resting motor threshold (RMT) was determined using single-pulse stimulation over the right primary motor cortex (M1) and systematically moving and adjusting until each pulse resulted in an isolated movement of the right thumb at rest (abductor pollicis brevis muscle). The machine output was adjusted to the lowest intensity that reliably induced thumb movement [15]. The RMT was defined as the lowest stimulus intensity could elicit at least five twitches in abductor pollicis brevis muscle (Motor Evoked Potentials) out of ten consecutive stimuli given over the “motor hot spot” at M1.
During real and sham stimulations, the TMS coil was aligned in a parasagittal line (stimulation coil was held at a 45° angle off the midline, with the handle pointing in the posterior direction) 5 cm (right DLPFC) from the area that produced right abductor pollicis brevis muscle movement for resting motor threshold testing [8] (Fig. S2). Stimulation was given at a pulse frequency of 1.0 Hz over the right DLPFC at 90% of resting motor threshold. rTMS was given in 8 trains of 150 pulses/train at inter train interval of 1 min. Total of 1200 stimuli were given during each session which lasted for 27 min. There were 5 sessions (Monday through Friday) per week and rTMS therapy was given over 4 consecutive weeks (20 sessions). During Sham (placebo/inactive) stimulation, an inactive rTMS coil (MC-B70, MagVenture, MagPro, Denmark) was used and placed over the same area as the active coil. The sham coil produced similar sound as the real coil but without active stimulation of the brain.
Primary outcome
The primary outcome was defined as a reduction in pain intensity. This was assessed with the help of Numerical Pain Rating Scale (NPRS), an 11-point numerical scale ranging from ‘0’ representing “No pain” to ‘10’ representing “Pain as bad as you can imagine” or “Worst pain imaginable” [16].
Secondary outcomes
We assessed Pain related depression, anxiety, impact of pain and quality of life, as secondary outcomes using McGill Pain Questionnaire (MPQ) [17]; Hamilton Depression Rating Scale (HDRS) [18]; Hamilton Anxiety Rating Scale (HARS) [19] and WHOQOL-Quality of Life-BREF (WHOQOL-BREF) questionnaire [20]. The other secondary outcomes assessed were, the NFR, pain modulation (effect of cold pressor test; CPT on NFR) and estimation of oxidative stress markers.
In the present study, NFR was recorded according the method described by Willer and colleagues [21]. Further details of the NFR are mentioned in our research article [22] (Fig. S3). Pain modulation was assessed by observing the effect of cold pressor test (CPT) on NFR parameters according to methods shown by Sandrini et al. [23] (Fig. S4). For biochemical estimation, blood samples were collected in heparinzed BD21q vacutainer collection tubes. The samples were centrifuged at 1500 rpm for 15 min at 4°C. The plasma was separated and transferred to the microcentrifuge tube and stored at − 80 °C till further analysis. Urine samples were also collected and stored at − 80 °C until further analysis. Both, blood and urine samples were collected at two time points i.e. pre rTMS and immediately post rTMS. To assess the oxidative stress, plasma levels of TBARS (QuantiChromTM TBARS Assay Kit (DTBA-100), USA) and urinary levels of F2-isoprostane (Elabscience Biotechnology Co. Ltd. (Elabscience®, China) were estimated with commercial kits.
Statistical analysis
All the analyses were performed using IBM SPSS Statistics version 22.0 (IBM Statistics for Windows, Chicago, IL, USA) and graphs were created using Graph Pad Prism 5.01 for Windows, (GraphPad Software, San Diego, California, USA). Baseline scores of the parameters between FMS Sham-rTMS and FMS Real-rTMS groups were compared using paired t test/ Wilcoxon Signed Rank test. The difference between FMS-Sham and FMS-rTMS was analyzed using unpaired t test/ Mann-Whitney U test. p < 0.05 was considered statistically significant. Analysis of follow- up data was done by paired t tests/ Wilcoxon Signed Rank test examining follow-up scores relative to post-rTMS (immediate after completion of therapy). Repeated Measures ANOVA was applied to assess the statistical significance within the group between two subsequent time points after Bonferroni correction.
Results
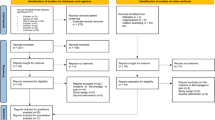
After Real/Sham rTMS, Follow-up (FU) was done at three time points i.e. 15 days post-rTMS (FU-1), 3 months post-rTMS (FU-2) and 6 months post-rTMS (FU-3). At the end of follow-up, 41 subjects in Sham-rTMS while 45 subjects in Real-rTMS group were analysed (Fig. S5). The data is presented as Mean ± SD and/or Median (25Q-75Q). Age, height, body weight and other general body parameters were comparable between Sham and Real-rTMS group (Table 1).
Primary outcome
A decrease in NPRS ratings due to rTMS treatment was observed in the Real-rTMS group immediately post-rTMS (p = 0·001) which was sustained through 6 months (Fig. 1). In the Sham-rTMS group no significant change in pain ratings was observed.
: Effect of rTMS on subjective pain rating scores in FMS. Subjective pain assessed by numerical pain rating scale (NPRS) in Sham (n = 41) and Real-rTMS (n = 45) group at each time points;pre, post and follow up (FU-1 = 15 days, FU-2 = 3 months and FU-3 = 6 months post-rTMS). Hash (#) symbol indicates within group p value. Asterisk (*) symbol indicates p value between Sham-rTMS and Real-rTMSgroup. *p or #p < 0.05; **p or ##p < 0.01; ***p or ###p < 0.001
Secondary outcomes
The ratings of affective-motivational components of pain were reduced post-rTMS (p = 0·001) and were maintained even at 6 months when compared to its baseline value (Table 2). The results of other components of MPQ ratings have been presented in Table 2. The HDRS and HARS ratings of Real-rTMS group decreased, post-rTMS and the changes were maintained through 6 months of therapy (Figs. 2 and 3). WHOQOL-BREF ratings were significantly increased post-rTMS when compared to baseline in Real-rTMS and through 6 months of therapy (Table 2).
: Effect of rTMS on pain related depression in FMS. Depression assessed by Hamilton depression rating scale (HRDS) in Sham (n = 41) and Real-rTMS (n = 45) group at each time points;pre, post and follow up (FU-1 = 15 days, FU-2 = 3 months and FU-3 = 6 months post-rTMS). Hash (#) symbol indicates within group p value. Asterisk (*) symbol indicates p value between Sham-rTMS and Real-rTMS group. *p or #p < 0.05; **p or ##p < 0.01; ***p or ###p < 0.001
: Effect of rTMS on pain related anxiety in FMS. Anxiety assessed by Hamilton anxiety rating scale (HARS) in Sham (n = 41) and Real-rTMS (n = 45) group at each time points;pre, post and follow up (FU-1 = 15 days, FU-2 = 3 months and FU-3 = 6 months post-rTMS). Hash (#) symbol indicates within group p value. Asterisk (*) symbol indicates p value between Sham-rTMS and Real-rTMSgroup. *p or #p < 0.05; **p or ##p < 0.01; ***p or ###p < 0.001
In Real-rTMS group, the threshold (volts, V) of NFR post-rTMS and 15 days post-rTMS was higher, compared to the baseline (p = 0·001) as well as from the Sham-rTMS group (Fig. 4). No significant change was observed in other parameters of NFR (latency, duration and amplitude) between Sham-rTMS and Real-rTMS group (Table 3).
: Effect of rTMS on spinal nociception (NFR) in FMS. Central nociception assessed by nociceptive flexion reflex (NFR) thresholds in Sham (n = 41) and Real-rTMS (n = 45) group at each time points;pre, post and follow up (FU-1 = 15 days, FU-2 = 3 months and FU-3 = 6 months post-rTMS). Hash (#) symbol indicates within group p value. Asterisk (*) symbol indicates p value between Sham-rTMS and Real-rTMS group. *p or #p < 0.05; **p or ##p < 0.01; ***p or ###p < 0.001
A sustained improvement in NFR thresholds (V) in response to cold noxious stimulus (4-5 ºC) during DNIC paradigm was observed in Real-rTMS group post-rTMS which was maintained 15 days post-rTMS also compared to the baseline (Table 3). NFR latency (ms), amplitude (μV) and duration (ms) did not change significantly in response to CPT (DNIC paradigm) in Real-rTMS or Sham-rTMS group during post-rTMS and 15 days post-rTMS. Plasma levels of TBARS were comparable in Real-rTMS group at baseline and post-rTMS (Real) (p = 0·12). Urinary levels of F2-isoprostane levels, did not vary significantly between groups at baseline and post-rTMS (Table 3).
Side effects due to sham/real rTMS
During the entire study period no serious side effects of rTMS were observed. However, two Real-rTMS allocated patients complained of headache; two Sham-rTMS allocated patients complained of neck pain while another patient reported mild dizziness.
Discussion
Selection of low frequency right DLPFC rTMS
In the present study, low-frequency rTMS (1 Hz) of right dorsolateral prefrontal cortex was selected as low frequency is safer and, previous studies targeting DLPFC have shown its beneficial effects in relieving chronic pain and depression in fibromyalgia [8, 25]. According to an earlier research, chronic pain is known to be closely associated with depression and anxiety, in fact the disability felt by the patient due to chronicity of pain and the existing poor quality of life, could be a contributory cause of depressive symptoms [26]. A reduction in both pain and depression/anxiety has been observed by targeting (reducing the activity) of DLPFC via transcranial direct current stimulation [27]. Low frequency TMS causes long-lasting inhibition of cell–cell communications or long term depression; whereas high-frequency TMS can produce long term potentiation [28, 29]. Neuro-imaging studies have also affirmed that the DLPFC may have a role in top–down mode of inhibition through descending fibres from the prefrontal cortex (PFCx) which may modulate pain perception [30, 31]. The findings of an fMRI research suggested that interhemispheric DLPFC connectivity can affect pain tolerance by altering interhemispheric inhibition [26]. Low frequency rTMS stimulation of right DLPFC possibly causes the removal of transcallosal inhibition, allowing an enhanced descending inhibition from the left hemisphere [10].
Effect of rTMS on subjective pain assessment
We observed a significant decrease in the pain ratings (NPRS) in Real-rTMS group compared to Sham-rTMS group which was sustained till 6 months of follow up period. Our findings are consistent with previous studies which reported reduced pain ratings after rTMS therapy and showed that rTMS performed substantially better than placebo, in management of FMS [25]. Pain related depression and anxiety ratings were also reduced in Real-rTMS group, this is consistent with the previous reports of right DLPFC TMS studies in chronic pain [8, 25]. We also recorded an improvement in pain scores in the Sham-rTMS though not statistically significant suggesting some placebo effect may exist for such therapies. Similar results have been reported with placebo type sham manoeuvres [32]. Our findings are supported by recent study from our group which has assessed the effect of low frequency rTMS on the right DLPFC in chronic tension type headache and reported a beneficial effect on pain and related symptoms [10].
Effect of rTMS on objective pain measures and pain modulation
Ours is amongst the first few studies to report the effect of rTMS on threshold of NFR. NFR is a spinally mediated withdrawal response that has been used to assess pain objectively. NFR was recorded at baseline and post rTMS to assess the pain objectively. In our FMS patients NFR thresholds were significantly lower indicating an exaggerated response to painful stimuli or hyperalgesia [22]. However, at the end of real-rTMS treatment, there was an increase in NFR threshold which was maintained during follow-up i.e. 15 days post-rTMS. Considering the intricacy of the procedure and time involved, NFR was not recorded at the 3 and 6 months follow-up time points. In contrast, a previous study which administered a single rTMS session, observed no significant effect of rTMS on threshold or recruitment curve of NFR in healthy subjects [33]. The contrasting results may be due to difference in study population and the rTMS protocol (single session of rTMS). The mechanisms that are thought to contribute to the pain-relieving effects of rTMS in experimentally induced and chronic pain lie within the medial or the lateral pain pathways and the descending pain inhibitory systems, modulating the pain perception [34].
In our study, descending pain inhibitory pathways were assessed by recording the effect of CPT on NFR. In our patients, pain tolerance time to noxious cold water was lower indicating diffuse hyper-excitability and sensitization of the nociceptive system which is also shown by earlier reports on fibromyalgia [35]. After rTMS therapy, we found an increase in threshold of NFR during CPT and pain tolerance for CPT suggesting that rTMS has alleviated the diffuse hyper-excitability and sensitization of the nociceptive system associated with FMS. Findings of this study are consistent with previous research suggesting that low-frequency (1 Hz) rTMS of the right DLPFC could increase cold pain tolerance [36]. Our results of pain modulation paradigm are also in accordance with a recent study on chronic myofascial pain syndrome which found that rTMS enhanced the cortico-spinal inhibitory system (41.74% reduction in quantitative sensory testing and conditioned pain modulationand a decrease of 23.94% in the intracortical facilitation) [37].
rTMS and oxidative stress markers
Recently, oxidative stress has been implicated as an important factor in the pathogenesis of FMS [11]. Although, we did not find any difference between levels of oxidative stress markers (TBARS and F2-isoprostane) before and after rTMS. To the best of our knowledge, no published report is available on effect of rTMS on TBARS and F2-isoprostane in FMS. The literature offers limited information about oxidative stress in FMS. It has been reported that malondialdehyde (MDA), which is an indicator of lipid peroxidation, increased [11, 12] and vitamin E, which prevents lipid peroxidation, is decreased in FMS patients [11].
Proposed mechanism of action rTMS in fibromyalgia
The possible mechanism of relief in pain and associated depression and anxiety after DLPFC stimulation could be through top-down modulation by rTMS. As DLPFC is associated with other brain areas such as rostral anterior cingulate cortex (which has been known to play an important role in pain processing [3] and bilateral amygdala and contralateral anterior insula (where an increased neuronal activity is observed in association with depressive symptoms) [38], it can potentially modulate this nexus. Recent studies have also observed decrease in regional cerebral blood flow (rCBF) in the bilateral medial prefrontal cortex, bilateral premotor area, bilateral anterior insula, right anterior and posterior insula, left anterior cingulate, right subgenual cingulate and right frontal cortex after right DLPFC rTMS. These studies have also reported a correlation between therapeutic efficacy of rTMS and decreased rCBF in bilateral frontal white matter [39,40,41,42]. Another study reported a significant decrease of activation in the bilateral middle frontal gyrus with right DLPFC-rTMS in rTMS responders [43]. Therefore, improvement in depression and anxiety after therapy suggests that rTMS of right DLPFC positively influences the brain regions responsible for such symptoms.
In our study, improvement in quality of life with rTMS indicates that right DLPFC stimulation may also have positive effect on the limbic system (right medial temporal cortex, involved in modulation of the emotional aspects of pain) [44] and superior temporal sulcus (involved in the social cognition and perception underlying social functioning of quality of life) [45,46,47], as neural connections have been reported between these areas and the limbic system [48].
The mechanisms that are thought to contribute to the pain relieving effects of rTMS of the motor cortex in experimentally induced pain and in chronic pain are the modulation of pain perception within the medial or the lateral pain pathway and the modulation of the descending inhibitory systems [34, 49]. In the present study, increased NFR threshold during CPT and the enhanced pain tolerance (during CPT) suggest that rTMS of DLPFC indirectly modulates nociception through its association with the cingulate cortex and medial thalamus which are known to be involved in nociceptive modulation [50,51,52]. The DLPFC is a highly integrated area with the “affective circuit” [53] i.e. ventral and anterior division of the anterior cingulate cortex including the hypothalamus, amygdala, orbitofrontal cortex, nucleus accumbens, and other limbic structures [54]. Therefore, the positive effects of rTMS on pain and associated symptoms are probably due to modulation of right DLPFC which further modulates the brain areas related with this ‘affective circuit’ (Fig. 5).
: Putative mechanism for the effect of low frequency right DLPFC in fibromyalgia syndrome. Proposed mechanism for effect of Rt DLPFC rTMS on pain and related symptoms of FMS. (rDLPFC = right dorsolateral prefrontal cortex; rVMPFC = right ventromedial prefrontal cortex; ACC = anterior cingulate cortex; OFC = orbitofrontal cortex; rCBF = regional cerebral blood flow). ‘Skull with TMS coil’ image was adapted from Diana et al., [24]
To sum up, decrease in ratings of pain and related symptoms, increased NFR threshold and the enhanced pain tolerance during CPT suggests that rTMS of DLPFC modulates nociception, associated with the cingulate cortex and medial thalamus which are known to be involved in such type of nociceptive modulation [50, 51].
Strengths and limitations of the study
There are many strengths of our study. Based on the published literature on the effect of rTMS in FMS symptoms, this is the first study which shows long term beneficial effects of rTMS (6 months follow-up). Most of the previous rTMS studies have reported 1–2 weeks of follow-up, and some have also followed their patients till 1 month or 3 months. Moreover, in earlier studies number of rTMS sessions were less as compared to our rTMS protocol and continuity of the sessions was also lacking in these studies [25, 45].
This is the first study which has tediously explored the effect of low frequency right DLPFC rTMS on subjective and objective pain parameters, descending pain modulation and oxidative stress markers in FMS patients.
Although, we have extensively studied the role of rTMS applied over DLPFC on pain status in FMS, yet there are some limitations that need to be addressed. We identified hotspot for rTMS therapy by using abductor muscle twitch. This method of getting hotspot has limitations , as far as targeting the actual area of stimulation is concerned. Recent research has recommended MRI based neuro-navigational techniques to localize the DLPFC thereby decreasing the inter-subject variability [55]. The exact mechanism of action of low frequency on pain modulation and central neurotransmitters involved could not be addressed. This could be due to inter-subject variability. Our study is a single blinded trial, although the participants’ blinding was meticulous, yet the rTMS administrator bias cannot be disregarded.
Future directions
Further studies should investigate the consistency of structural and functional DLPFC abnormalities in chronic pain conditions utilizing neuroimaging techniques; compare low frequency right DLPFC rTMS with other administration protocols or other novel paradigms in FMS patients. Future research should also assess the effect of rTMS on the levels of central neurotransmitters which play predominant role in endogenous pain facilitatory and inhibitory pathways. For oxidative stress markers, more studies may be designed with assessment at multiple time points during progression and post therapy, including follow-up period in FMS patients.
Conclusions
Findings of our 6-months follow-up study suggest that right DLPFC rTMS can significantly reduce pain and associated symptoms of FMS and preferentially targets spinal pain circuits and top-down pain modulation with no effect on oxidative stress markers.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and additional information can be provided if requested.
References
Cabo A, Cerdá G, Trillo J. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med Clin. 2017;149(10):441–8.
Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 2007(Vol. 36, no. 6, pp. 339-356). WB Saunders.
Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. PAIN®. 2009;144(1–2):95–100.
Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010;149(3):495–500.
Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsøe B, Dincer F, Henriksson C, Henriksson KG. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536–41.
Thomas AW, Graham K, Prato FS, et al. A randomized, doubleblind,placebo-controlled clinical trial using a low-frequencymagnetic field in the treatment of musculoskeletal chronic pain. Pain Res Manag. 2007;12:249–58.
Brakemeier EL, Wilbertz G, Rodax S, Danker-Hopfe H, Zinka B, Zwanzger P, et al. Patterns of response to repetitive transcranialmagnetic stimulation (rTMS) in major depression: Replicationstudy in drug-free patients. J Affect Disord. 2008;108:59–70.
Sampson SM, Rome JD, Rummans TA. Slow-frequency rTMS reduces fibromyalgia pain. Pain Med. 2006;7(2):115–8.
Martin L, Borckardt JJ, Reeves ST, Frohman H, Beam W, Nahas Z, et al. A pilot functional MRI study of the effects of prefrontal rTMS on pain perception. Pain Med. 2013;14(7):999–1009.
Mattoo B, Tanwar S, Bhatia R, Tripathi M, Bhatia R. Repetitive transcranial magnetic stimulation in chronic tension-type headache: a pilot study. Indian J Med Res. 2019;150(1):73.
Akkus S, Naziroglu M, Eris S, Yalman K, Yilmaz N, et al. Levels of lipid peroxidation, nitric oxide, and antioxidant vitamins in plasma of patients with fibromyalgia. Cell BiochemFunct. 2009;27:181–5.
Cordero,M.D. Oxidative stress in fibromyalgia: pathophysiology and clinical implications. ReumatologíaClínica (English Edition), 7(5) (2011) 281–283.
Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, George MS. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152(11):2477–84.
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–10.
Borckardt JJ, Nahas Z, Koola J, George MS. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J ECT. 2006;22(3):169–75.
Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55:195–203.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56.
Hamilton MA. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5.
Skevington SM, Lotfy M, O’Connell KA. WHOQOL group. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310.
Willer JC, Bathien N. Pharmacological modulations on the nociceptive flexion reflex in man. Pain. 1977;3(2):111–9.
Tanwar S, Mattoo B, Kumar U, Bhatia R. Can aberrant spinal nociception be a marker of chronicity of pain in fibromyalgia syndrome? J Clin Neurosci. 2019;65:17–22.
Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP, Nappi G. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26(7):782–9.
Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18(11):685.
Lee SJ, Kim DY, Chun MH, Kim YG. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham-controlled trial with 1-mo follow-up. Am J Phys Med Rehabil. 2012;91(12):1077–85.
Sevel LS, Letzen JE, Staud R, Robinson ME. Interhemispheric dorsolateral prefrontal cortex connectivity is associated with individual differences in pain sensitivity in healthy controls. Brain Connect. 2016;6:357–64.
Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8–12.
Bear MF. Homosynaptic long-term depression: a mechanism for memory? Proc Natl Acad Sci U S A. 1999;96:9457–8.
Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–4.
Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(5):1079–91.
Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron. 2002;35(2):383–93.
Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinicalconditions. Cochrane Database Syst Rev. 2010;1:1–3.
Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. PAIN®. 2009;147(1–3):224–32.
Tamura Y, Okabe S, Ohnishi T, Saito DN, Arai N, Mochio S, Inoue K, Ugawa Y. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107(1–2):107–15.
Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302.
Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Núñez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cogn Brain Res. 2005;25(1):153–60.
Dall’Agnol L, Medeiros LF, Torres IL, Deitos A, Brietzke A, Laste G, Caumo W. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain. 2014;15(8):845–55.
Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52(5):1577–84.
Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58(1):29–36.
Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin Neurosci. 2011;65(2):175–82.
Kito S, Hasegawa T, Okayasu M, Fujita K, Koga Y. A 6-month follow-up case report of regional cerebral blood flow changes in treatment-resistant depression after successful treatment with bilateral transcranial magnetic stimulation. J ECT. 2011;27(1):e12–4.
Kito S, Hasegawa T, Koga Y. Cerebral blood flow ratio of the dorsolateral prefrontal cortex to the ventromedial prefrontal cortex as a potential predictor of treatment response to transcranial magnetic stimulation in depression. Brain Stimul. 2012;5(4):547–53.
Fitzgerald PB, Sritharan A, Daskalakis ZJ, De Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27(5):488–92.
Peyron R, García-Larrea L, Grégoire MC, Costes N, Convers P, Lavenne F, Mauguière F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(9):1765–80.
Boyer L, Richieri R, Faget C, Padovani R, Vaillant F, Mundler O, Lançon C, Auquier P, Guedj E. Functional involvement of superior temporal sulcus in quality of life of patients with schizophrenia. Psychiatry Res Neuroimaging. 2012;202(2):155–60.
Giovagnoli AR, Franceschetti S, Reati F, Parente A, Maccagnano C, Villani F, Spreafico R. Theory of mind in frontal and temporal lobe epilepsy: cognitive and neural aspects. Epilepsia. 2011;52(11):1995–2002.
Moulier V, Gaudeau-Bosma C, Isaac C, Allard AC, Bouaziz N, Sidhoumi D, Braha-Zeitoun S, Benadhira R, Thomas F, Januel D. Effect of repetitive transcranial magnetic stimulation on mood in healthy subjects. Socioaffect Neurosci Psychol. 2016;6(1):29672.
Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–12.
Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–25.
Chai SC, Kung JC, Shyu BC. Roles of the anterior cingulate cortex and medial thalamus in short-term and long-term aversive information processing. Mol Pain. 2010;6(1):42.
Russo JF, Sheth SA. Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain. Neurosurg Focus. 2015 Jun 1;38(6):E11.
Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Textbook of pain. 5th ed. Burlington, Massachusetts, USA: Elsevier Health Sciences; 2005. p. 125–42.
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25.
Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118.
Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015;8(5):965–73.
Acknowledgements
Authors acknowledge FMS patients for participation and technical staff of Pain Research and TMS laboratory, AIIMS, New Delhi, India.
Funding
Research work was supported by University Grant Commission (UGC), New Delhi. SumanTanwar was awarded by UGC-Junior/Senior Research fellowship grant for her doctoral research by UGC, New Delhi, India.
Author information
Authors and Affiliations
Contributions
Renu Bhatia takes responsibility for the integrity of the work as a whole, from inception of idea to final manuscript. Suman Tanwar, Uma Kumar and Renu Bhatia made primary contributions in study design, data acquisition, analysis and interpretation of data and preparing of the final manuscript. Bhawna Mattoo made notable contributions in data acquisition, analysis, interpretation and preparing of the final manuscript along with its critical revision. All authors have discussed the results and gave final approval of the version to be submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted at the Pain Research and TMS Laboratory, Department of Physiology, All India Institute of Medical Sciences (AIIMS) New Delhi, India. Human Ethics committee of the AIIMS, New Delhi (Ref No: IESC/T-251/15.06.2013) approved the research protocol in 2013. The study was also registered in ICMR-CTRI; India (Ref No: CTRI/2013/12/004228). All procedures performed during study involving human participants were in accordance with the ethical standards of the Human Ethics committee of the AIIMS, New Delhi. All the participants provided written informed consent. All the participants were enrolled only after getting ethical approval.
Consent for publication
Consent to publish was also obtained from all the participants included in the study.
Competing interests
Authors declare no competing interests concerning the work reported in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Study protocol. Figure S2. Patient receiving rTMS therapy. Figure S3. Nociceptive Flexion Reflex recording set up. Figure S4. Set up for DNIC study (NFR recording during cold pressor test; CPT). Figure S5. CONSORT flow diagram
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanwar, S., Mattoo, B., Kumar, U. et al. Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: a randomised controlled trial with 6-months follow up. Adv Rheumatol 60, 34 (2020). https://doi.org/10.1186/s42358-020-00135-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-020-00135-7