Abstract
Background
Toxicities of Vernonia amygdalina and Tithonia diversifolia leaf powders and extracts on larva and adult mortality of Dermestes maculatus on smoke-dried catfish were evaluated in the laboratory. The leaf powders were admixed at 1, 3, 6, 9 and 12 g/100 g of smoked catfish in 500 ml plastic container while plant extracts were tested at 1, 3, 6, 9 and 12% concentrations.
Results
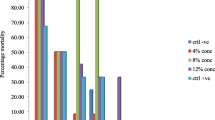
The toxicities of the plant products were concentrations and exposure time dependent. Significant difference (P < 0.05) existed between the toxicity of V. amygdalina and T. diversifolia leaf powders and control. Tithonia diversifolia powder evoked 27.5, 40, 52.7, 60 and 82.5% larval mortalities at the various concentrations after 24 h of exposure of the catfishes to the plant powder. Similarly, V. amygdalina leaf powders caused 20, 30, 42.5, 52.5 and 77.5% larval mortalities at varying concentrations of 24 h intervals of exposure to the plant powder. Tithonia diversifolia powder achieved 100% mortality at 9 g dosage after 96 h of treatment. Similar trend was achieved on the response of both plant leaf extracts as protectants of smoke-dried catfish against hide beetle.
Conclusion
Tithonia diversifolia powder and extract were more lethal than bitter leaf (V. amygdalina) and could be integrated as smoked fish protectant against hide beetle (D. maculatus).
Similar content being viewed by others
Background
Fish is widely acceptable on the menu table of most inhabitant of the earth irrespective of socio-economic status, age and religious background, and it constitutes about 50% of total animal protein needed for growth (Adesina et al. 2014; Ileke et al. 2020a). Fish protein contains essential amino acids such as lysine, methionine and isoleucine, these are relatively deficient in other animal proteins (Abolagba et al. 2011; Adesina et al. 2014; Ileke et al. 2020b). Fish is also rich in proteins such as riboflavin and thiamine. It contains vitamins A, B, D, E and K as well as some useful minerals (Yem et al. 2006). Fish contains polysaturated fatty acids which reduces blood cholesterol in the system thereby lowering blood pressure in hypertension patient (Okunade 2011; Ileke et al. 2020a, b).
Fresh fishes depend solely on smoking and drying as a means of preservation among the local fish farmers in Nigeria (Adesina et al. 2014). However, the smoked fish is vulnerable to insect damage from the family Dermestidae and order Coleoptera (Adedire 2001). The activities of the hide beetle can lead to changes in fish appearance, powdering as a result of larval instar growth rendering it unfits for feasting and selling (Adesina et al. 2014, 2016). Insect pests of fish can transmit Escherichia coli when the moisture content is high due to high proliferation of the hide beetle. This usually gives room for bacteria and mould growth (Jackson and Ayub 2013; Jose and Adesina 2014). Some of the losses caused by D. maculatus infestation are enormous, and these include physical loss that reduces the quantity of fish available for human consumption, economic loss which affects the monetary value of the infested smoked fish. Also, the nutritional loss which results in physical and economic losses thereby increases the retail value of fish beyond the purchasing power of the poor (Adesina et al. 2014).
Effectiveness of synthetic chemical insecticides against weevils, beetles and moths of cereals, legumes and tubers have been documented by many researchers (Ileke 2019; Ileke et al. 2020c, d; Obembe et al. 2020). Nevertheless the adoption of this method to reduce physical, economic and nutritional losses caused by insect pests of smoked fish has not been fully accepted by farmers and consumers. This rejection is due to high toxicity of chemicals to consumers, irritating odour and residual effect on the environment couple with high cost of purchase (Amusan and Okorie 2002; Odeyemi et al. 2000; Ahmed et al. 2013; Ileke et al. 2020a); toxicity to non-targeted organisms, environmental health hazard and slow biodegradation of active compounds (Ileke et al. 2020b;). This research is focusing on the use of plants derived insecticides that is environmental friendly and readily available in our immediate environment. Many entomologists and postharvest fish scientists have reported the efficacy of powders, ash and extracts from the leaf, stem bark, flower, seed and root of many plants to suppress oviposition, prevent adult emergence and reduced fish quality and quantity losses (Adedire and Lajide 2000; Amusan and Okorie 2002; Anyaele and Amusan 2003; Onu and Baba 2003; Fasakin 2003; Adebote et al. 2007; Akinwumi 2011; Abdullahi et al. 2012; Ahmed et al. 2013; Jose and Adesina 2014; Adesina et al. 2014, 2016; Ileke et al. 2020a, 2020b).
Tithonia diversifolia is a species of flowering plant commonly called marigold that belongs to a family Asteraceae. The height is about 2–3 m (6.6–9.8 ft) with ligneous stalks in form of woody shrubs. Marigold, a weed that grows fast and has become an alternative to an expensive synthetic fertilizer (Jama et al. 2000). Its presence in soil can lead to high plant yields and improve soil nutrients (Jama et al. 2000). Vernonia amygdalina originated in Africa and also belongs to the family Asteraceae (Odugbemi 2006). It can be regarded as a shrub or small tree as it can grow between 2 and 5 m in height with leaves that are green in colour, petiolate and elliptically shaped of about 6 mm diameter (FAO 2001). The characteristic odour and bitter taste of the leaves are due to the presence of saponins, tannins, alkaloids and glycosides. Vernonia amygdalina is popularly referred to as bitter leaf (Ologunde et al. 1992; Bonsi et al. 1995). The specific objectives of this research are to evaluate V. amygdalina and T. diversifolia leaf powders and extracts as toxicants against larva and adult stages of skin beetle (D. maculatus) on smoke-dried catfish (C. gariepinus).
Methods
Experimental location
The study was conducted in the Research Laboratory of the Department of Environmental Biology and Fisheries, Adekunle Ajasin University, Akungba Akoko (AAUA) at mean temperature and relative humidity of 28.9 °C and 76.4%, respectively.
Collection and preparation of Tithonia diversifolia and Vernonia amygdalina leaves
Fresh V. amygdalina and T. diversifolia leaves were collected from a farm at Ipinsa, Akure, Ondo State, Nigeria. These plant leaves were brought into the Research laboratory (Department of Plant Science and Biotechnology, Adekunle Ajasin University Akungba Akoko, Nigeria) for identification by a Plant Taxonomist. The plant leaves were washed thoroughly with clean water, air-dried in the laboratory for three weeks. Each of the plant leaves was pulverized separately into fine powder using an electric blender. The powders were further sieved to pass through 1mm2 perforation. The fine powder was kept separately inside an air tight sample containers in the refrigerator to retain their freshness before application.
Extraction of experimental plants
About 300 g of T. diversifolia powders were soaked in an extraction bottle containing 600 ml of absolute ethanol for 3 days. The dissolved powders were stirred intermittently in order to ensure uniformity in extraction. A double layer Whatman No. 1 filter papers were used for the extraction process. The mixture of the solvent and the extract was separated by means of rotary evaporator (Udo 2011). The resulting extracts were then air-dried to remove any remaining solvent. The same procedure was adopted for the extraction of V. amygdalina. The extracts were kept separately in labelled plastic bottles till when needed. Different extract concentrations of 1%, 3%, 6%, 9% and 12% were prepared separately (Ashamo and Akinnowonu 2012).
Collection and preparation of catfish
Fresh catfishes weighing between 400 and 500 g were collected from Hatchery Unit, AAUA. They were smoked locally, free of common salt and seasoning. The smoked fishes were sterilized by re-heating at 40 °C for one hour in a hot air oven (Gallenkamp Oven) in the laboratory in order to kill any developmental stages (egg, larva, pupa and adult) of the insect pests that may be present (Adesina et al. 2016), and allowed to cool at room temperature in the laboratory in order to prevent mouldiness (Adedire et al. 2011).
Insect culture
The initial skin beetle (D. maculatus) culture used for this study were obtained from an infested smoke-dried catfish collected from smoked fish sellers at Ibaka Market, Akungba Akoko, Ondo State, Nigeria. The skin beetles were introduced into one litre plane glass kilner jar containing 1.5 kg of smoked catfish free of salt and seasoning obtained from Fish sellers. Jar containing the beetles was covered with muslin cloth and placed in an insect rearing cage. Newly emerged larvae (0–3 days) were removed from stock culture and placed on fresh uninfected smoked fish (Adesina et al. 2016). Adults were removed after 14 days of oviposition (egg laying) period. Water was supplied by soaking cotton wool in water (Odeyemi and Daramola 2000). The first filial generation (F1) was used for the skin beetle experiment.
Hide beetle bioassay
Evaluation of V. amygdalina and T. diversifolia powders on D. maculatus larvae
Toxicities of V. amygdalina and T. diversifolia leaf powders on D. maculatus larvae were assessed by admixed separately at concentrations 1 g, 3 g, 6 g, 9 g and 12 g/100 g of smoked catfish in 500 ml plastic container. Vernonia amygdalina and T. diversifolia powders were thoroughly mixed with the smoked fish. Treated smoked fish and untreated (control) were infested with twenty (20) newly emerged first instar larvae about 0–3 day old of hide beetle, replicated four times and laid out in Complete Randomized Block Design in insect cage. Larval mortality was assessed and recorded every 24 h for 96 h. Abbott (1925) method of correcting mortality of larva was adopted as follows:
where PT = corrected mortality (%), PO = observed mortality (%), PC = control mortality (%).
The larva bioassay setup was kept inside the insect rearing cage and daily observations were made until adult emergence. The number reaching adult stages was recorded and expressed as percentage adult emergence (Odeyemi and Daramola 2000).
Inhibition rate (IR) of adult emergence was evaluated using the method described by Tapondju et al. (2002).
where Cn—Total number of teneral adult insects in control. Tn—Total number of teneral adult insects treated in smoked fish.
Weight loss of the smoked catfish was expressed in percentage by re-weighing after 35 days and the percentage loss in weight was determined (Odeyemi and Daramola 2000).
Evaluation of V. amygdalina and T. diversifolia powders on D. maculatus adults
Toxicities of V. amygdalina and T. diversifolia leaves powders on D. maculatus adults were assessed by admixed separately at concentrations 1 g, 3 g, 6 g, 9 g and 12 g/100 g of smoked catfish in 500 ml plastic container. Vernonia amygdalina and T. diversifolia powders were thoroughly mixed with the smoked fish. Treated smoked fish and untreated (control) were infested with ten (10) newly emerged adults (0–3 day old), replicated four times and laid out in Complete Randomized Block Design in insect cage. Adult mortality was assessed and recorded every 24 h for 96 h. Abbott (1925) method of correcting mortality of larvae was adopted.
Evaluation of V. amygdalina and T. diversifolia ethanolic extracts on D. maculatus larvae
Toxicities of V. amygdalina and T. diversifolia extracts on D. maculatus larvae were assessed by admixed separately at concentrations 1%, 3%, 6%, 9% and 12%/100 g of smoked catfish in 500-ml plastic container. Vernonia amygdalina and T. diversifolia extracts were thoroughly mixed with the smoked fish. Treated smoked fish and untreated (control) were infested with 20 newly emerged 1st instar larvae between 0 to 3 day old of hide beetles, replicated four times and laid out in Complete Randomized Block Design in insect cage. Larval mortality was assessed and recorded every 24 h for 96 h. The larval bioassay setup was kept inside the insect rearing cage and daily observations were made until adult emergence. The number reaching adult stages was recorded and expressed as percentage adult emergence (Odeyemi and Daramola 2000). Inhibition rate (IR) of adult emergence was identified using the method described by Tapondju et al. (2002). Weight loss of the smoked catfish was determined by re-weighing after 35 days and the percentage loss in weight was determined.
Evaluation of V. amygdalina and T. diversifolia extracts on D. maculatus adults
Toxicities of V. amygdalina and T. diversifolia leaves extracts on D. maculatus adults were assessed by admixed separately at concentrations 1%, 3%, 6%, 9% and 12%/100 g of smoked catfish in 500 ml plastic container. Vernonia amygdalina and T. diversifolia extracts were thoroughly mixed with the smoked fish. Treated smoked fish and untreated (control) were infested with 10 newly emerged adults between 0 and 3 day old, replicated four times and laid out in Complete Randomized Block Design in insect cage. Adult mortality was assessed and recorded every 24 h for 96 h.
Statistical analysis
Percentage mortality of larvae and adults was calculated and corrected using Abbott’s formula (Abbott 1925). Data were subjected to analysis of variance (ANOVA), and means were separated using the Tukey’s Test.
Results
Toxicities of V. amygdalina and T. diversifolia leaves Powder on Mortality of D. maculatus larvae
The toxicities of V. amygdalina and T. diversifolia powders were concentrations and exposure time dependent (Table 1). Tithonia diversifolia leaf powder was the most potent to the larvae of hide beetles (D. maculatus). There was significant difference (P < 0.05) between the toxicity effects of V. amygdalina, T. diversifolia powders and the control. Tithonia diversifolia powder evoked 27.5%, 40%, 52.7%, 60% and 82.5% mortalities of D. maculatus larvae after 24 h of exposure period at concentrations 1 g, 3 g, 6 g, 9 g and 12 g/100 g of smoke-dried catfish, respectively. Similarly, V. amygdalina powders caused 20%, 30%, 42.5%, 52.5% and 77.5% mortalities of D. maculatus larvae at concentrations 1 g, 3 g, 6 g, 9 g and 12 g/100 g of smoke-dried catfish, respectively. Tithonia diversifolia powder caused 100% mortality of hide beetle larvae at concentration 9 g/100 g of smoked catfish after 96 h of treatment while at treatment with V. amygdalina, the full mortality was observed after the same exposure period (96 h.) by using higher concentration from the leaf powder (12 g/100 g of smoked catfish).
Toxicities of V. amygdalina and T. diversifolia Powders on % Inhibition, weight loss adult Emergence of D. maculatus
The number of catfish, D. maculatus adults (F1) that emerged from treated infested fish with D. maculatus larvae was significantly lower (P < 0.05) than in the control (Table 2). There was no adult emergence and weight loss in smoked catfish treated with V. amygdalina and T. diversifolia leaves powders at rate 6 g, 9 g and 12 g/100 g of smoke-dried catfish. The number of surviving larvae from treated smoked fish with V. amygdalina and T. diversifolia leaf powders decreased as plant powder concentration increased. The percentage inhibition rate at all concentrations for the V. amygdalina and T. diversifolia leaf powders increased significantly (P < 0.05) compared with the control. The least inhibition rate of 76.47% in progeny development was observed in catfish treated with 1 g/100 g of smoke-dried catfish of V. amygdalina leaf powders. The weight loss observed in samples of fish treated with 1 g of V. amygdalina and T. diversifolia powders was significantly lower (P < 0.05) than that of the control.
Toxicities of T. diversifolia and V. amygdalina Powders on Mortality of D. maculatus adults
Vernonia amygdalina and T. diversifolia leaf powders at tested concentrations were potent to the adult hide beetle; D. maculatus (Table 3). There was a significant difference (P < 0.05) between the effect of each plant leaf powder and the control. Tithonia diversifolia powder was the most potent which caused 22.5%, 37.5%, 50%, 57.5% and 80% mortalities of adult skin beetle, D. maculatus at rates of 1 g, 3 g, 6 g, 9 g and 12 g/100 g of smoke-dried catfish after 24 h of treatment. This was followed by bitter leaf powders which evoked 12%, 27.5%, 40%, 50% and 72.5% of adult mortalities of skin beetle at the above-tested concentrations of smoked catfish after 24 h of treatment. Tithonia diversifolia powder achieved 100% death of adult D. maculatus at all tested concentrations after 96 h of exposure. Mortality of adult skin beetle (D. maculatus) increased with increase in length of treatment and concentrations used.
Toxicities of V. amygdalina and T. diversifolia Extracts on Mortality of D. maculatus larvae
Tithonia diversifolia leaf extract was the most potent to the larvae of skin beetles; D. maculatus (Table 4). There was substantial difference (P < 0.05) between the toxicities of V. amygdalina, T. diversifolia extracts and the control. Tithonia diversifolia extract caused 50%, 62.5%, 80%, 92.5% and 100% larval mortalities at concentrations of 1%, 3%, 6%, 9% and 12%/100 g of smoke-dried catfish after 24 h of exposure, respectively. Similarly, V. amygdalina leaf extract caused 40%, 60%, 77.5%, 90% and 97.5% larval mortalities of D. maculatus at concentrations of 1%, 3%, 6%, 9% and 12% / 100 g of the smoke-dried catfish after 24 h of exposure to plant extract, respectively. Tithonia diversifolia extract caused 100% death of skin beetle larvae at concentration of 3%/100 g of the smoked catfish after 96 h of treatment. Similarly, V. amygdalina evoked 100% death of hide beetle larvae at concentration of 6%/100 g of smoke-dried catfish after 96 h of treatment.
Toxicities of V. amygdalina and T. diversifolia Extracts on percentage Inhibition, weight loss adult Emergence of D. maculatus
The number of catfish (D. maculatus) adults (F1) that emerged from treated fish infested with larvae of D. maculatus was significantly lower (P < 0.05) than in the control (Table 5). There was no adult emergence and weight loss in smoke-dried catfish treated with V. amygdalina and T. diversifolia leaf extracts at rates of 1%, 3%, 6%, 9 g% and 12%/100 g of the smoked fish. The number of surviving larvae from treated smoked fish with V. amygdalina and T. diversifolia leaf extracts decreased with increased in concentration of the plant extracts. The percentage inhibition rate at all concentrations for the V. amygdalina and T. diversifolia leaf extracts increased significantly (P < 0.05) when compared to the control. The weight loss observed in samples of fish treated with 1 g of V. amygdalina and T. diversifolia extracts was significantly lower (P < 0.05) than that of control.
Toxicities of V. amygdalina and T. diversifolia Extracts on Mortality of D. maculatus adults
Vernonia amygdalina and T. diversifolia leaf extracts at the tested concentrations were toxic to the adult hide beetle, D. maculatus (Table 6). There was a significant difference (P < 0.05) between the effect of each plant powder and the control. Tithonia diversifolia extract was the most toxic which caused 47.5%, 60%, 77.5%, 90% and 100% mortality of adult hide beetle (D. maculates) at the different concentration tested of smoked catfish after 24 h of treatment. This was followed by bitter leaf, V. amygdalina extract (Table 6). Tithonia diversifolia extract caused 100% death of adult D. maculatus at concentration of 12%. Mortality of adult skin beetle (D. maculatus) increased with increase in length of treatment and concentrations used.
Discussion
Insect and fish postharvest scientists were equipped with many procedures to screened botanicals for their efficiency in management of coleopteran insect pests such as hide beetle (Dermestes maculatus) that infested smoke-dried catfish during storage (Amusan and Okorie 2002; Odeyemi et al. 2000). In any method employed, a potent material is adversely toxic to insect developmental stages (eggs, larvae, pupae and adults). It also serves as oviposition deterrent or preventing the full expression of its oviposition through antifeedant, repellence, attractant for contact poisoning (Akinkurolere 2012).
Toxicity of T. diversifolia and V. amygdalina powders and extracts on larval and adult mortality of hide beetle (Dermestes maculatus) on smoked dried catfish was revealed in this research study. Tithonia diversifolia powder achieved 100% death of hide beetle larvae at concentration of 9 g/100 g of smoked catfish after 96 h of treatment. Adedire and Akinneye (2004) reported on the effectiveness of T. diversifolia in the control of cowpea beetle (Callosobruchus maculatus). The authors reported that T. diversifolia powder was lethal at high dosages and exposure time dependent. The higher the concentration, the higher the mortality rate. The results also validated the report of Adoyo et al. (1997) who found Tithonia to be toxic to termites in a farm in Busia district of Kenya. The toxicity of the Tithonia could be attributed to the presence of two sesquiterpene lactones, seven germacranolides and four eudesmanolides isolated from T. rotundifolia (Bohlmann et al. 1984). Isolation of a novel dinorxanthane sesquiterpene called diversifolide [4, 15-dinor-3-hydroxy-1 (5)-xanthen—12, 8-olide], a new chromone and four other known compounds from the root of T. diversifolia by Chen and Lin (1999). These compounds may be responsible for its insecticidal actions (Adedire and Akinneye 2004).
Vernonia amygdalina powder and extract were also toxic to larvae and adults skin beetle. Vernonia amygdalina powder evoked 100% mortality of hide beetle larvae at concentration of 12 g/100 g of smoked catfish after 96 h of treatment. The extract evoked 100% mortality of hide beetle larvae at concentration of 6%/100 g of the smoked catfish after 96 h of treatment. The results obtained in this work is in agreement with many earlier researchers on the utilization of bitter leaf as entomocides (Ileke 2015; Adedire and Lajide 2003; Musa et al. 2009; Moses and Dorathy 2011). Ileke (2015) reported on protectability of V. amygdalina against cowpea beetle (Callosobruchus maculatus) infesting cowpea seeds. Adedire and Lajide (2003) reported the effectiveness of V. amygdalina in the control of maize weevil (Sitophilus zeamais). Musa et al. (2009) reported the effectiveness of V. amygdalina in the management of cowpea bruchid. Moses and Dorathy (2011) who reported efficacious of bitter leaf over garlic and ginger as the most toxicant that protected cowpea seeds against cowpea weevil (C. maculatus).
Toxicity of V. amygdalina powder and extract on larvae and adults of D. maculatus could be as a result of the presence of some chemical compounds like alkaloids and sesquiterpene lactones which contain and 11, 13-dihydrovernodalin, vernodalol and vernodalin (Pascual et al. 2001). These compounds contain antifeedant and repellent properties and also act as ovicidal, larvicidal and adulticidal (Pascual et al. 2001).
Bitter leaf (V. amygdalina) and T. diversifolia powders and extracts prevented the emergence of D. maculatus adults in the treatment against larval and adult stages. This result is in agreement with the observation of Jose and Adesina (2014). The powders and extracts of V. amygdalina and T. diversifolia adversely affected survival and growth of skin beetle. Deterrent properties of plants could lead to growth inhibition in insects (Akhtar and Isman 2004).
The development of D. maculatus larvae was inhibited in all the treated smoked catfishes compared to the control. This agreed with earlier scientists who have reported on the bioefficacy of plant products as contact insecticides on D. maculatus (Fasakin 2003; Adebote et al. 2007; Akinwumi 2011; Jose and Adesina 2014). The significant reduction in progeny development could be as a result of higher mortality of D. maculatus larva which in turn results in lower adult emergence. The powders and extracts of the tested plants were more lethal to the larval than the adult stage of hide beetle.
Conclusion
The results of this study clearly revealed that powders and extracts of the tested plants were more lethal to larval than adult stage. The larvae were more mobile and feed voraciously than adults (Adedire 2001). Tithonia diversifolia powder and extract were more toxic than V. amygdalina and can be integrated as smoked fish protectant against skin beetle (D. maculatus). The experimental plants were readily and widely available in all ecological zone in Nigeria. I therefore recommend it adoption for fish farmers or traders for the management of D. maculatus infestation during processing, transportation and storage of smoke-dried catfish (C. gariepinus).
Availability of data and materials
Data collected and analysed during the current study are available from the corresponding author on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Eco Entomol 18:265–267
Abdullahi N, Ubayi SM, Babura SR (2012) Determination of the effect of Zingiber officianale and Allium sativum powder on the mortality of Dermestid maculatus larvae on treated dried Claris gariepinus fish. Inter J Appl Res and Tech 1(3):129–133
Abolagba OJ, Igene JO, Usifoh CO (2011) Studies of Pesticide Residues in Smoked Catfish (Clarias Gariepinus) in Nigeria: Some Health Implications. Aust J Basic and Appl Sci 5(5):496–502
Adebote DA, Abolude DS, Oniye SJ, Olododo SS, Hassan MM (2007) Larvicidal and repellant action of Detarium microcarpum seed oil against larvae of D lardarius Coleoptera: Dermestidae in dried Clarias gariepinus fish. J Entmol. 3:248–253
Adedire CO, Akinneye JO (2004) Biological activity of tree marigold, Tithonia diversifolia, on cowpea seed bruchid, Callosobruchus maculatus (Coleoptera: Bruchidae). Ann Appl Biol 144:185–189
Adedire CO, Lajide L (2000) Effect of pulverized plant materials on fish damage and growth performance of the fish beetles Dermestes maculatus (Degeer). Entomol Soc Nig Occ Publ 32:215–221
Adedire CO, Lajide L (2003) Ability of extract of ten tropical plant species to protect maize grains against infestation by the maize weevil Sitophilus zeamais during storage. Nig J Exp Biol 4(2):175–179
Adedire CO, Obembe OM, Akinkurolere RO, Oduleye SO (2011) Response Callosobruchus maculatus Fabricius (Coleoptera: Chrysomelidae: Bruchidae) to extracts of cashew kernels. J Plt Dis Prot 118(2):75–79
Adesina JM, Jose AR, Adetuyi OO, Olorunfemi DA (2014) Larvicidal activity of Phyllanthus fraternuspowder in suppressing Dermestes maculatusDegeer (Coleoptera: Dermestidae) infestation on smoked Africancatfish (Clarias gariepinus). Intern J Aquacul. 4(11):67–72
Adesina JM, Jose AR, Rajashekar Y (2016). Bio-efficacy of Clerodendrum capitatum (Willd) Schumachet. Thonn (Lamiales: Verbenaceae) against Dermestes maculatus De Geer, 1774 (Coleoptera: Dermestidae) larvae infestation on smoked catfish Claria gariepinus (Burchell, 1822) (Siluriformes: Clariidae). Braz J Biol Sci 3(5):37–44.
Adoyo F, Mukalama JB, Enyola M (1997) Using Tithonia concoctions for termite control in Busia district. Kenya ILEIA Newslett 13:24–26
Ahmed H, Ahmed KN, Khanom Noor P (2013) Damage Potential and control measures of Necrobia rufipes(De Geer) (Coleoptera: Cleridae) on dry fish with plant materials. Bangl J Sci Ind Res. 48(1):19–24
Akhtar Y, Isman MB (2004) Comparative growth inhibitory and anti-feedant effect of plant extract and pure allelochemical on four phytophagus insect species. J Appl Entomol 128(1):32–38
Akinkurolere RO (2012) Comparative effects of three plant powders and pirimiphos-methyl against the infestation of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in Cowpea Seeds. SOAJ Entomol Stud. 1:87–99
Akinwumi FO (2011) Evaluation of some plant materials for the control of smoked fish pest, Dermestes maculatus degeer (Coleoptera: Dermestidae) In Clarias pariepinus Burchell (Pisces: Clariidae). ARPN J Agricul Biol Sci 6(7):65–69
Amusan AAS, Okorie TG (2002) The use of Piper guineense fruit oil (PFO) as protectant of dried fish against Dermestes maculatus (De Geer) infestation. Glob J Pur Appl Sci 8:197–201
Anyaele OO, Amusan AAS (2003) Toxicity of hexanolic extract of Dennettia tripetala Piper guineense Schum and Thonn against Dermestes maculatus Degeer (Coleoptera: Dermestidae) and Necrobia rufipes Degeer (Coleoptera: Cleridae) on dried fish. Nig J Entomol 18:109–117
Ashamo MO, Akinnawonu O (2012) Insecticidal efficacy of some plant powders and extracts against the Angoumois moth, Sitotroga cerealella (Olivier) [Lepidoptera: Gelechiidae]. Arch Phytopath Cr Prot 45(9):1051–1058
Bohlmann F, Jakupovic J, Muller CL, Schuster A (1981) Naturally occurring terpene derivatives, Part 353—Part 352: A. Rustaiyan, C. Zdero, and F. Bohlmann. Phytochem. 20(3):292–293. https://doi.org/10.1002/anie.198102921
Bonsi MLK, Osuji PO, Tuah AK, Umunna NN (1995) Vernonia amygdalina as a supplement to teff straw (Eragrosis tef) fed to Ethiopian Menz sheep. Agroforest Sys. 31:229–241
Chen Y, Lin K (1999) Radical polymerization of styrene in the presence of C6o. J Polym Sci Part A 37(15):2969–2975
FAO (2001) Vernonia amygdalina. Food and Agriculture Organization (FAO). 2001; Retrieved from http://www.fao.org/livestock/agap/frg/Visit/Ida/Vernonia%20amygdalina.htm on the 29th of November, 2019
Fasakin EA (2003) Resistance status of some commercially smoked fish species to storage insect pest, Dermestes maculatus Deeger in the tropics. Nig J Appl Bio 4(1):41–45
Ileke KD (2015) Entomotoxicant potential of Bitter leaf, Vernonia amygdalina powder in the control of cowpea bruchid, Callosobruchus maculatus (Coleoptera: Chrysomelidae) infesting stored cowpea seeds. Octa J Envir Res 3(3):226–234
Ileke KD (2019) The efficacy of alstonia boonei stembark oil as a long-term storage protectant against cowpea bruchid, callosobruchus maculatus (fab.) (Coleoptera: Chrysomelidae). Jord J Biol Sci. 12(3):329–337
Ileke KD, Adesina JM, Abidemi-Iromini AO, Abdulsalam MS (2020a) Entomocide effect of Alstonia boonei De Wild on Reproductive Performance of Dermestes maculatus (Coleoptera: Dermestidae) Infestation on Smoked Catfish Claria gariepinus (Pisces: Clariidea). Int J Tropical Insect Sci. https://doi.org/10.1007/s42690-020-00321-6
Ileke KD, Ojo DO, Obembe OM, Ogunbiyi YK (2020b) Susceptibility of hide beetle, Dermestes maculatus(De Geer) [Coleoptera: Dermestidae] to powder and extract of two species of capsicum fruits onsmoke-dried catfish, Clarias gariepinus(Burchell) [Pisces: Clariidae]. Egyptian Acad J Biol Sci A Entomol 13(3):97–111
Ileke KD, Idoko JE, Ojo DO, Adesina BC (2020c) Evaluation of botanical powders and extracts from Nigerian plants as protectants of maize grains against maize weevil, Sitophilus zeamais (Motschulsky) [Coleoptera: Curculionidae]. Biocat Agricul Biotech 27:101702. https://doi.org/10.1016/j.bcab.2020.101702
Ileke KD, Adesina JM, Nwosu LC, Olagunju A (2020d) Perforation index assessment of cowpea seeds against Cowpea Bruchid, Callosobruchus maculatus (Fab.) [Coleoptera: Chrysomelidae] infestation using Piper guineense. J Basic Appl Zool 81(60):1–10
Jackson HOO, Ayub VOO (2013) Effect of salting on houseflies (Musca domestica) and beetles (Necrobia rufipes and Dermestes maculatus) Infestation of Fish from Lake Victoria, Kenya. Inter J Res Pur Appl Microbio 3(1):30–35
Jama B, Palm CA, Buresh RJ, Niang A, Gachengo C, Nziguheba G (2000) Tithonia diversifolia as a green manure for soil fertility improvement in western Kenya: A review. Agroforest Sys 2002(49):202
Jose AR, Adesina JM (2014) Larval SUSCEPTIBILITY OF Dermestes maculatus (Degeer, 1776) (Coleoptera Dermestidae) to Secamone afzelii (Schult) K Schum leaf powder on smoke-dried fish. Inter J Aquacul 4(17):102–107
Moses O, Dorathy O (2011) pesticidal effect of some plant materials for the control of weevils (Callosobruchus maculatus) in some varieties of cowpea during storage in makurdi, Southern Guinea Agro-ecological zone of Nigeria. Entomol Soci Nige. In: 42nd annual conference Ibadan book of abstracts, 20 p.
Musa AK, Oyerinde AA, Owolabi FO (2009) Evaluation of the efficacy of mixed leaf powders of Vernonia amygdalina L. and Ocimum gratissimum against Callosobruchus maculatus. Acad J Ent. 2(2):85–87
Obembe OM, Ojo DO, Ileke KD (2020) Efficacy of kigelia Africana (Lam.) Benth. Plant extracts on cowpea seed beetle, Callosobruchus maculatus Fabricius [Coleoptera: Chrysomelidae] affecting stored cowpea seeds Vigina unguiculata. Heliy 6:05215
Odeyemi OO, Daramola AM (2000) Storage practices in the tropics. Dave Collins Publication, Nigeria, p 235
Odeyemi OO, Owode RA, Akinkurolere A (2000) Toxicity and population suppression effects of Parkia clappertomiana on dried fish pests (Dermestes maculatus and Necrobia rufipes). Glob J Pur Appl Sci 6(2):191–195
Odugbemi TT (2006) Outline and pictures of medicinal plants from Nigeria. University of Lagos Press, Lagos
Okunade OA (2011) Fish: The Healthy Friendly species. In: Kolo RJ, Orire AM (eds) In: Proceedings of the 26th annual conference of the fisheries society of Nigeria held federal university of technology, Minna, Nigeria from 28th November–2nd December, 2011. pp 25–34
Ologunde MO, Ayorinde FO, Shepard RK, Afolabi OA, Oke OL (1992) Sterols of seed oils of Vernonia galanesis, Amaranthus cruentus, Amaranthus caudatus, Amaranthus hybrids and Amaranthus hypochondriacus growth in the humid tropics. J Fd Agric 58:221–225
Onu I, Baba GO (2003) Evaluation of neem products Azadirachta indica A. Juss for control of Dermestid beetle D. maculatus (Coleoptera:Dermestidae) in dried fish. Nig J Ent. 20:105–115
Pascual M, Slowing K, Carretero E, Sánchez Mata D, Villar A (2001) Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol 76:201–214
Tapondju LA, Alder A, Bonda H, Fontem DA (2002) Efficacy of powder and oil from Chenpodium ambrosioides leaves as post-harvest grain protectants against six stored products beetles. J Stor Prod Res 38:395–402
Udo IO (2011) Potentials of Zanthoxylum xanthoxyloides (LAM) for the control of stored product insect pests. J Stor Prod Postharv Res. 2(3):40–44
Yem IY, Sanni AO, Musa YM (2006) The role of fish production in food security and nutrition in Nigeria. J Sci Ind Stud 3(2):23–27
Acknowledgements
I thank Dr. Abiodun Adeyemi Eniade of the Hatchery Unit, Department of Environmental Biology and Fisheries, AAUA for his assistance during fish processing. I also thank Dr. O. A. Obembe of the Department of Plant Science and Biotechnology for the authentication of the plants used.
Funding
The author declares that no fund was received from anybody in carrying out this study. This research study was funded solely by the author.
Author information
Authors and Affiliations
Contributions
KDI conceived, designed the study, collected data, search references and manuscript write up. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ileke, K.D. Responses of two plant-derived bioinsecticides as protectants of smoke-dried catfish, Clarias gariepinus [Pisces: Clariidae] against hide beetle, Dermestes maculatus (De Geer) [Coleoptera: Dermestidae]. Bull Natl Res Cent 45, 28 (2021). https://doi.org/10.1186/s42269-020-00470-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-020-00470-1