Abstract
Background
The genus Albizia (Leguminoseae) is used in folk medicine for the treatment of a wide range of ailments. Recently, saponins from plant origin have attracted much attention. Saponins are recorded to have a broad range of biological and pharmacological activities. This study was performed to evaluate the protective role of Albizia chinensis bark methanolic extract (MEAC) against the genotoxicity induced by cyclophosphamide (CP) using different mutagenic parameters.
Results
The results showed that MEAC induced an inhibitory effect against chromosomal aberrations of CP in mouse bone marrow and spermatocytes. Such effect was found to be significant (p < 0.01) with a dose of 100 mg/kg treated once for 24 h and also after repeated treatment at a dose of 25 mg/kg for 7 days. In sperm abnormalities, the protective effect of Albizia extract showed a dose-related relationship. Different doses of MEAC (25, 50, and 100 mg/kg) significantly (p < 0.01) ameliorated sperm abnormalities induced by CP dose-dependently. The percentage of sperm abnormalities was decreased to 5.14 ± 0.72 in the group of animals treated with CP plus MEAC (100 mg/kg) indicating an inhibitory effect of about 50%.
Conclusion
MEAC at the doses examined was non-genotoxic compared to control (negative) and exhibited a protective role against CP genotoxicity.
Similar content being viewed by others
Background
The genus Albizia (Leguminoseae) comprises about 150 species; most of them are trees and shrubs inherent to tropical and subtropical regions of Africa and Asia (Migahid 1989). Several species of Albizia are utilized in traditional folk medicine for the treatment of many diseases such as stomach trouble, rheumatism, insomnia, anxiety, depression, and inflammatory disorders. Albizia members are also used as estrogenic, antipyretic, and analgesic agents and for treatment of swelling, wounds, and fractures (Chaudhary et al. 2011). The antimicrobial activity of some flavonoids which obtained from an Egyptian collection of A. chinensis was reported (Ghaly et al. 2010). Also, A. lebbeck has been chained to be useful in the treatment of Alzheimer’s and Parkinson’s diseases (Sanjay 2003).
Concerning the anti-carcinogenicity of Albizia species, it was recorded that A. chinensis stem bark extract has cytotoxic activity against a small panel of human cancer cell lines (Liu et al. 2009, 2010). The crude extract from A. chinensis was also reported to induce anti-proliferative activity against human oral (KB) and cervical (Hela) cancer cell lines (Manosroi et al. 2015). The Julibroside J8 which is a new extract separated from Albizia julibrissin showed varying degrees of anti-proliferative activity in human cancer cell lines (HeLa, PC-3MIE8, BGC-823, Bel-7402, MDA-MB-435, and LH-60) in vitro (Zou et al. 2005; Zheng et al. 2006). In addition, Albizia lebbeck methanolic extract increases the inhibitory effect towards MCF-7 in vitro. Such an effect was dose-dependent (Aditya et al. 2014). Also, Jangwan et al. (2010) demonstrated that A. lebbeck possess a potent cytotoxic effect against human squamous cell carcinoma HSC-3 and HSC-2 at IC50 (3.1 μg/mL and 4.2 μg/mL), respectively.
Cyclophosphamide is an effective anti-cancer drug widely used in the treatment of many types of cancers, e.g., acute and chronic leukemia, lymphomas, multiple myeloma, breast cancer, ovarian cancer, neuroblastoma, and sarcoma (Zaki et al. 2003). It is also used in immunosuppression disorders (Fahmy et al. 2015). In spite of its wide range of medical benefits, it can induce many destructive effects on patients under its treatment (Khan et al. 2014). Cyclophosphamide (CP) has the ability to induce genetic alterations (Ahmadi et al. 2008; Leal et al. 2012). It was used in the current study as a positive genotoxic agent. It was utilized for the evaluation of anti-mutagenic/anti-genotoxic efficacy of natural compounds and other chemicals (Sharma and Agrawal 2015; Mohamed and Aly 2018; Fahmy et al. 2019). So, the present work was designed to assess the protective role of the methanol extract of Albizia chinensis (MEAC) against the genotoxicity induced by CP.
Methods
Experimental animals
Mature male white Swiss mice (Mus Musculus), aged 9–12 weeks, were used in all experiments. The animals were obtained from a closed random-bred colony at the National Research Centre (Egypt). The mice used for each experiment were selected from mice of similar age (± 1 week) and weighing 20–25 g (± 2 g). Animals were housed in polycarbonate boxes with steel wire tops and bedded with wood shavings. Ambient temperature was controlled at 22 ± 3 °C with a relative humidity of 50 ± 15% and a 12-h light/dark photoperiod. Food and water were provided ad libitum.
Chemicals
Cyclophosphamide (CP) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in extraction were purchased from ADWIC (Cairo, Egypt).
Plant material
The bark of A. chinensis was collected from the zoological garden in Giza, Egypt, in June 2017. Plant identification was confirmed by Mrs. T. Labib, head specialist for plant identification in El-Orman public garden. The herbarium voucher specimen (No. 125) was deposited in the Herbarium of NRC (CAIRC).
Plant extract
The bark of A. chinensis (1.5 kg) was air-dried and powdered then defatted by soaking in n-hexane three times using 5-L solvent for each time. After drying, the plant material was extracted at room temperature with methanol until exhaustion. The combined methanolic extract was evaporated under vacuum to give a brown residue (350 g) which was kept in the refrigerator until use.
Treatment and experimental design
Chromosomal abnormalities in the bone marrow and mouse spermatocytes
A total of 45 mice were used. Mice were divided into nine groups of five animals each. The different groups were treated as follows: group I (negative control), group II (positive control, in which animals were i.p. injected with a single dose of CP at 20 mg/kg), groups III and IV (mice were treated with a single oral treatment of MEAC (100 mg/kg) and repeated doses for 7 days at 25 mg/kg (control plant)), groups V–VII (mice received a single dose of MEAC at 25, 50, and 100 mg/kg (orally) + CP (i.p)), groups VIII and IX (mice received oral treatment with MEAC at 25 mg/kg (3 and 7 successive days) + CP (single at the last day of treatment)). Animals were sacrificed 24 h after the last treatment. For the preparation of somatic and germ cell chromosomes, animals from the different groups were i.p. injected with colchicine (10 mg/kg) 2–3 h before sacrifice.
Sperm shape abnormalities
Six groups were taken (5 each—a total of 30 animals): group I (negative control), group II (positive control), group III (mice were treated with MEAC (100 mg/kg)), and groups IV–VI (mice received MEAC at 25, 50, and 100 mg/kg (orally) + CP (i.p)). All groups were treated for 3 consecutive days, and samples were taken 35 days after the 1st treatment.
Cytological preparations
Chromosomal aberration
Chromosomal preparation from the bone marrow was made according to the technique developed by Doherty et al. (2012). In brief, mouse bone marrow cells were collected from both femurs, and cells were incubated in hypotonic solution (KCL 0.075 M) for 20 min at 37 °C and then centrifuged. The cell pellets were resuspended in a fixative (methanol/glacial acetic acid). This step was repeated; then, resuspended cells in fixative were spread onto frozen slides, air-dried, stained with 10% Giemsa for 40 min, washed, and air-dried again. A hundred of well-spread metaphases were analyzed per animal for structural and numerical aberrations.
For chromosomal preparations from mouse spermatocytes, the method of Hassan et al. (2006) was followed. Every 100 well-spread metaphases were analyzed per mouse describing the different kinds of abnormalities. Scoring was performed under a × 2500 magnification light microscope (Litz, Germany).
Sperm shape abnormalities
Sperm were prepared according to the recommended method described by Fahmy et al. (2015). The epididymides excised and minced in isotonic sodium citrate solution (2.2%) dispersed and filtered to exclude large tissue fragments. Smears were stained with 1% eosin Y. A group of five mice was taken for each treatment, and a total of 1000 sperm were counted per animal; scoring different types of sperm abnormalities, head and tail abnormalities were recorded under a × 1000 magnification light microscope.
Statistical analysis
Data were computerized and analyzed using the Statistical Package of Social Sciences (SPSS Inc., version 20, Armonk, New York, IBM Corp.). One-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison test was used to determine the difference among the means. The level of statistical significance was set at p < 0.01.
Evaluation of the effect of MEAC to inhibit DNA damage induced in the CP group was carried out according to Madrigal-Bujaidar et al.’s (1998) equation as follows:
Inhibitory index (II) = [1 − (combined groups − control)/(CP − control)] × 100.
Results
Our results showed that a single dose of 100 mg/kg MEAC which represents the highest tested dose and the repeated doses for 7 days with 25 mg/kg were safe on somatic and germ cell chromosomes, and their effect was statistically non-significant compared to the control negative (Tables 1 and 2). A detailed study on the ameliorative effect of different doses of MEAC (25, 50, and 100 mg/kg) after a single treatment against chromosomal abnormalities of cyclophosphamide was recorded. The effect of repeated treatment with a dose of 25 mg/kg for 3 and 7 days was also determined. The results showed that MEAC induced an inhibitory effect against chromosomal aberrations of CP in mouse bone marrow and spermatocytes. Such effect was found to be significant (p < 0.01) with a dose of 100 mg/kg treated once for 24 h and also after repeated treatment at a dose of 25 mg/kg for 7 days. The percentage of chromosomal aberrations reached 14.00 ± 0.55 and 13.00 ± 0.65 in the bone marrow after excluding gaps compared with 19.20 ± 0.60 for CP alone. The percentage of aberrations reached 12.40 ± 0.58 and 11.60 ± 0.50 in mouse spermatocytes compared with 17.00 ± 0.54 for CP. Such effect represents an inhibition in the percentage of chromosome abnormalities by 33% and 39%. A dose and time relationship was described (Figs. 1 and 2).
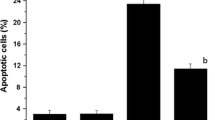
Concerning sperm abnormalities, the results showed that MEAC was safe at the highest tested dose, and its effect was nearly close to the negative control, where the percentage of morphological sperm abnormalities reached 2.26 ± 0.50 and 2. 34 ± 0.48. CP induced a highly significant percentage of sperm abnormalities compared to the control and plant. Such percentage reached 10.72 ± 0.56 which represents about 5-fold increases vs control value. The results also demonstrated that different doses of MEAC (25, 50, and 100 mg/kg) significantly (p < 0.01) ameliorated sperm abnormalities induced by CP dose-dependently (Fig. 3). The percentage of sperm abnormalities was decreased to 5.14 ± 0.72 in the group of animals treated with CP plus MEAC (100 mg/kg) indicating an inhibitory effect of about 50% (Table 3).
Discussion
The use of antineoplastic drugs is associated with many adverse side effects. These effects extended from general or organ-specific acute and chronic toxicity to DNA damage and even secondary tumor formation (Liu et al. 2014). One of the most attractive approaches to disease prevention involves the use of natural antioxidants (Manosroi et al. 2012). Herein, MEAC was tested for its protective role against DNA damage and mutagenesis induced by CP.
CP is one of the most widely used chemotherapeutic drugs. It is on the World Health Organization’s List of Essential Medicines and used for the treatment of malignant and non-malignant disorders (Hosseinimehr and Karami 2005; El-Husseiny et al. 2016). Cyclophosphamide is an alkylating agent that belongs to the nitrogen mustard family of medications. Its bioactivation by hepatic microsomal cytochrome P450 resulted in the formation of the active metabolites “phosphoramide mustard and acrolin.” Such metabolites can interfere with the duplication of DNA and the creation of RNA and induce a wide range of adverse side effects including genetic toxicity and reproductive impairments (Comish et al. 2014; Ince et al. 2014; Vredenburg et al. 2014). Overproduction of reactive oxygen species and lipid peroxidation was reported to be the major mechanisms in CP toxicity and may affect the activities of the main antioxidant enzymes (Lata et al. 2014).
The results of the present work revealed that CP induced strong chromosome damage in mouse bone marrow and spermatocytes which is statistically significant at p < 0.01. These results are in agreement with the findings of other authors who reported that CP is a strong inducer for chromosome aberrations, micronuclei, sister chromatid exchange, and rearrangements (Jain and Jain 2012; El-Souda et al. 2014, Fahmy et al. 2019). It also induced significant percentage of morphological sperm defects. This coincides well with the results of other authors (Tripathi and Jena 2008; Jalali et al. 2012; Fahmy et al. 2015).
The obtained results also showed that MEAC had a normal genotoxic effect as compared with the negative control. Conversely, it displayed a significant inhibitory effect against CP-induced chromosome damage at a dose level of 100 mg/kg treated once for 24 h and with a dose of 25 mg/kg treated for 7 days. The examination of sperm morphology showed that MEAC at the three tested concentrations reduced the CP-induced sperm abnormalities. The percentage of inhibition in sperm abnormalities was recorded as a function of MEAC concentrations.
Phytochemical investigation carried out on plants of the genus Albizia has revealed them as a good source of many active constituents. Triterpenoid saponins (Melek et al. 2007; Miyase et al. 2010) and flavonoids such as quercetin, kaempferol, and luteolin represent the main components (Ghaly et al. 2010). The triterpenoid saponin content of MEAC has been previously demonstrated (Liu et al. 2009, 2010). Saponins are naturally occurring substances widely distributed in plants and marine animals. In recent years, saponins from plant origin have attracted much attention. Saponins are recorded to have a broad range of biological and pharmacological activities, e.g., antiviral, immunomodulatory, anti-inflammatory, anti-mutagenic, cytotxic, and antitumor activity (Guang et al. 2014; Li et al. 2015; Wang et al. 2015). The anti-mutagenic activities of MEAC which are observed in the present work may be in part related to the presence of saponins. This opinion was supported by the previous reports of other authors: Scarpato et al. (1998) found that one of the saponins isolated from Bupleurum fruticosum had a dose-dependent inhibition of micronuclei induced by the anti-cancer drug mitomycin C in human lymphocytes. Also, the triterpenoid saponins isolated from involucral bracts of Cynara cardunculus L. showed anti-genotoxic activities against ofloxacin and acridine orange-induced DNA damage of chloroplast in Euglena gracilis (Krizková et al. 2004). The albizosides D and E which are triterpene saponins isolated from the stem bark of A. chinensis induced cytotoxic activity against the small panel of human cancer cell lines (Liu et al. 2010). Our results were also supported by the finding of EL-Hosry et al. (2014) who demonstrated that saxifragifolin B which is isolated from Cyclamen presicum and Cyclamen libanoticum prevented cells from mitomycin C genotoxicity, in addition to its safety for inducing micronuclei. It is also 37 and 56 times more active than mitomycin C against lung adenocarcinoma (NC1-H1299) and breast adenocarcinoma (SK-BR-3), respectively. Also, Melek et al. (2015) found that the total saponin fraction derived from Gleditsia caspica Desf had no genotoxic effect in mice. Although, it significantly alleviated chromosomal abnormalities induced by CP in mouse bone marrow and spermatocytes and reduced the viability of the human cancer cell line MCF-7 dose-dependently. In addition, the MEAC wood showed effective anti-proliferative activity against human cervical (Hela) and oral (KB) cancer cell lines (Manosroi et al. 2015). Saponins from ginseng stem-leaf showed strong antioxidant activity against oxidative stress induced by cyclophosphamide in chickens (Yu et al. 2015).
Flavonoids are considered one of the main bioactive constituents of A. chinensis (Ghaly et al. 2010). Such compounds possess a wide range of biological activities that have pharmacological and therapeutic interest. The ameliorative effect of MEAC that is demonstrated in the present work against CP genotoxicity may be related to the strong antioxidant properties of flavonoids which are intimately involved in the prevention of cellular damage. Rao (2002) mentioned that flavonoids possessed 4- to 5-folds of antioxidant activity as ascorbic acid. The antioxidant properties of flavonoids, the protection against DNA damage caused by various carcinogenic factors, and their role in genome stability are confirmed by other authors (George et al. 2016).
Quercetin, luteolin, and kaempferol are important flavonoids previously identified from A. chinensis (Melek et al. 2015). Quercetin was found to inhibit chronic myeloid leukemia KBM7 cells (Li et al. 2014) and to protect rat erythrocytes against oxidative stress and genotoxicity induced by the synthetic pyrethroid lambda-cyhalothrin (Abdallah et al. 2012). Min and Ebeler (2009) reported that quercetin has a strong antioxidant capacity that protects DNA damage both by reducing the oxidative stress and enhancing the DNA repair mechanism. Luteolin showed potent anti-carcinogenic and anti-mutagenic activities against dietary carcinogens (Seelinger et al. 2008; Orhan et al. 2013). Strong antioxidant, anti-inflammatory, and antitumor activities of luteolin were also recorded (Kasala et al. 2016; Kang et al. 2017; Xiong et al. 2017). Also, Kaempferol was reported to exhibit a broad spectrum of beneficial bioactivities, including antioxidant, anti-mutagenic, anti-inflammatory, and chemopreventive potential (Rocha et al. 2016; Choi et al. 2017).
Conclusion
To the best of our knowledge, these data describe for the first time the anti-mutagenic activity of MEAC. In the present study, CP was genotoxic in somatic and germ cells evidenced by a remarkable increase in chromosomal aberrations in the bone marrow and mouse primary spermatocytes and its induction of sperm morphology deformities. The literature review showed that overproduction of reactive oxygen species and lipid peroxidation was the major mechanism in CP toxicity and DNA damage. The presence of pharmaceutical active ingredients, triterpenoid saponins, and flavonoids, in MEAC play a significant protective role against CP genotoxicity. These natural compounds have very potent antioxidant activities against DNA damage and oxidative stress induced by CP.
Availability of data and materials
Data are available from the authors on reasonable request.
Abbreviations
- MEAC:
-
Albizia chinensis bark methanolic extract
- CP:
-
Cyclophosphamide
- ANOVA:
-
Analysis of variance
References
Abdallah FB, Fetoui H, Fakhfakh F, Keskes L (2012) Caffeic acid and quercetin protect erythrocytes against the oxidative stress and the genotoxic of lambda-cyhalothrin in vitro. Human and Experimental Toxicology 31(1):92-100. https://doi.org/10.1177/0960327111424303
Aditya SJ, Naresh KL, Mokkapati A (2014) Evaluation of in vitro cytotoxicity of Andrographis paniculata, Duranta serratifolia and Albizzia lebbeck whole plant extracts by MTT assay against MCF-7 and HT-29 cell lines. Current Research in Microbiology and Biotechnology 2:351–353
Ahmadi A, Hosseinimehr SJ, Naghshvar F, Hajir E, Ghahremani, M (2008) Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch Pharm Res 31 (6):794–797. https://doi.org/10.1007/s12272-001-1228-z
Chaudhary A, Kaur P, Kumar N, Singh B, Awasthi S, Lal B (2011) Chemical fingerprint analysis of phenolics of Albizia chinensis based on ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry and antioxidant activity. Natural Product Communications 6 (11):1617-1620. https://doi.org/10.1177/1934578X1100601115.
Choi KC, Son YO, Hwang JM, Kim BT, Chae M, Lee JC (2017) Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharmaceutical Biology 55(1): 611-619. https://doi.org/10.1080/13880209.2016.1266673
Comish PB, Drumond AL, Kinnell HL, Anderson RA, Matin A, Meistrich ML, Shetty G (2014) Fetal cyclophosphamide exposure induces testicular cancer and reduced spermatogenesis and ovarian follicle numbers in mice. PLoS One 9(4): e93311. https://doi.org/10.1371/journal.pone.0093311.
El Hosry L, Di Giorgio C, Birer C, Habib J, Tueni M, Bun SS, Herbette G, De Meo M, Ollivier E, Elias R (2014) In vitro cytotoxic and anticlastogenic activities of saxifragifolin B and cyclamin isolated from Cyclamen persicum and Cyclamen libanoticum. Pharmaceutical Biology 52(9):1134-1140. https://doi.org/10.3109/13880209.2013.879600
El-Husseiny K, Motawei H, Ali MS (2016) Continuous low-dose oral cyclophosphamide and methotrexate as maintenance therapy in patients with advanced ovarian carcinoma after complete clinical response to platinum and paclitaxel chemotherapy. International Journal of Gynecological Cancer 26 (3), 437-442. https://doi.org/10.1097/IGC.0000000000000647
El-Souda SSE, Mohammed RS, Marzouk MM, Fahmy MA, Hassan ZM, Farghly AA (2014) Antimutagenicity and phytoconstituents of Egyptian Plantago albicans L. Asian Proc J Trop Dis 4 (2):S946-S951. https://doi.org/10.1016/S2222-1808(14)60764-7
Fahmy MA, Farghaly AA, Hassan EE, Hassan EM, Abdel-Samie NS, Abdel-Ghany EM, Omara EA (2019) Fennel (Foeniculum vulgare) essential oil ameliorates DNA and histopathological damage induced by cyclophosphamide in mice. Bioscience Research 16 (1):320-336. Print ISSN: 1811-9506 Online ISSN: 2218-3973
Fahmy MA, Hassan NH, El-Fikey SA, Elalfy HG (2015) A mixture of honey bee products ameliorates the genotoxic side effects of cyclophosphamide. Asian Pacific Journal Tropical Diseases 5:638-644. https://doi.org/10.1016/S2222-1808(15)60904-5
George VC, Dellaire G, Rupasinghe HP (2016) Plant flavonoids in cancer chemoprevention: role in genome stability. The Journal of Nutritional Biochemistry 45:1-14. https://doi.org/10.1016/j.jnutbio.2016.11.007.
Guang C, Chen J, Sang S, Cheng S (2014) Biological functionality of soyasaponins and soyasapogenols. Journal of Agricultural and Food Chemistry 62(33):8247-8255. https://doi.org/10.1021/jf503047a.
Hassan NHA, Fahmy MA, Farghaly AA, Hassan EES (2006) Antimutagenic effects of selenium and vitamins against the genotoxicity induced by cobalt chloride in mice. Cytologia 71(3): 2013-2222. https://doi.org/10.1508/cytologia.71.213.
Hosseinimehr SJ, Karami M (2005) Chemoprotective effects of captopril against cyclophosphamide–induced genotoxicity in mouse bone marrow cells. Archive of Toxicology 79(8):482–486. https://doi.org/10.1007/s00204-005-0655-7
Ince S, Kucukkurt I, Demirel HH, Acaroz DA, Akbel E, Cigerci IH (2014) Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 108:197-204. https://doi.org/10.1016/j.chemosphere.2014.01.038
Jain R, Jain SK (2012) Effect of Buchanania lanzan Spreng bark extract on cyclophosphamide induced genotoxicity and oxidative stress in mice. Asian Pac J Trop Med 5(3): 187-191. https://doi.org/10.1016/S1995-7645(12)60022-4
Jalali AS, Hasanzadeh S, Malekinejad H (2012) Achillea millefolium inflorescence aqueous extract ameliorates cyclophosphamide–induced toxicity in rat testis: stereological evidences. Chin J Nat Med 10(4): 247-254. https://doi.org/10.1016/S1875-5364(12)60050-8
Jangwan JS, Dobhal M, Kumar N (2010) New cytotoxic saponin of Albizia lebbeck. Indian Journal of Chemistry 49:123–126. URI http://hdl.handle.net/123456789/7143
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ, Hyun JW (2017) Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int J Oncol. 51(4):1169-1178. https://doi.org/10.3892/ijo.2017.4091
Kasala ER, Bodduluru LN, Barua CC, Gogoi R (2016) Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother 82:568-577. https://doi.org/10.1016/j.biopha.2016.05.042
Khan JA, Shahdad S, Makhdoomi MA, Hamid S, Bhat GM, Jan Y, Nazir S, Bashir Z, Banoo S (2014) Effect of cyclophosphamide on the microanatomy of liver of albino rats. Int J Res Med Sci 2(4):1466–1469. https://doi.org/10.5455/2320-6012.ijrms20141141
Krizková L, Mucaji P, Nagy M, Krajcovic J (2004) Triterpenoid cynarasaponins from Cynara cardunculus L. reduce chemically induced mutagenesis in vitro. Phytomedicine 11(7-8):673-678. https://doi.org/10.1016/j.phymed.2003.09.001
Lata S, Singh S, Nath Tiwari K, Upadhyay R (2014) Evaluation of the antioxidant and hepatoprotective effect of Phyllanthus fraternus against a chemotherapeutic drug cyclophosphamide. Applied Biochemistry and Biotechnology 173(8): 2163-2173. https://doi.org/10.1007/s12010-014-1018-8
Leal MF, Antunes LM, Lamarao MF, da Silva CE, da Silva ID, Assumpcao PP, Andrade EF, Rezende AP, Imbeloni AA, Muniz JA, Pinto GR, Smitha Mde A, Burbano RR (2012) The protective effect of canova homeopathic medicine in cyclophosphamide-treated non-human primates. Food Chem Toxicol 50:4412–4420. https://doi.org/10.1016/j.fct.2012.09.002
Li E, Sun N, Zhao JX, Sun YG, Huang JG, Lei HM, Guo JH, Hu YL, Wang WK, Li HQ (2015) In vitro evaluation of antiviral activity of tea seed saponins against porcine reproductive and respiratory syndrome virus. Antiviral Therapy 20(7):743–752. https://doi.org/10.3851/IMP2937
Li W, Zhao Y, Tao B, Zhang Y (2014) Effects of quercetin on hedgehog signaling in chronic myeloid leukemia KBM7 cells. Chinese Journal of Integrative Medicine 20(10):776–781. https://doi.org/10.1007/s11655-014-1817-3
Liu M, Hales BF, Robaire B (2014) Effects of four chemotherapeutic agents, bleomycin, etoposide, cisplatin, and cyclophosphamide, on DNA damage and telomeres in a mouse spermatogonial cell line. Biology of Reproduction 90 (4): 72. https://doi.org/10.1095/biolreprod.114.117754
Liu R, Ma S, Yu S, Pei Y, Zhang S, Chen X, Zhang J (2009) Cytotoxic oleanane triterpene saponins from Albizia chinensis. Journal of Natural Product 72(4):632-639. https://doi.org/10.1021/np800576s
Liu R, Ma SG, Liu YX, Yu SS, Chen XG, Zhang JJ (2010) Albizosides D and E, two new cytotoxic triterpene saponins from Albizia chinensis. Carbohydrate Research 345(13):1877-1881. https://doi.org/10.1016/j.carres.2010.05.024
Madrigal-Bujaidar E, Diaz Barriga S, Cassani M, Márquez P, Revuelta P (1998) In vivo and in vitro antigenotoxic effect of nordihydroguaiaretic acid against SCEs induced by methyl methanesulfonate. Mutat Res 419:163–168. https://doi.org/10.1016/S1383-5718(98)00128-4
Manosroi A, Akazawa H, Kitdamrongtham W, Akihisa T, Manosroi W, Manosroi J (2015) Potent antiproliferative effect on liver cancer of medicinal plants selected from the Thai/Lanna medicinal plant recipe database “MANOSROI III”. Evidence Based Complementary and Alternative Medicine 2015:397181. https://doi.org/10.1155/2015/397181
Manosroi J, Sainakham M, Manosroi W, Manosroi A (2012) Anti-proliferative and apoptosis induction activities of extracts from Thai medicinal plant recipes selected from MANOSROI II database. Journal of Ethnopharmacology 141(1):451-459. https://doi.org/10.1016/j.jep.2012.03.010
Melek FR, Aly FA, Kassem IA, Abo-Zeid MA, Farghaly AA, Hassan ZM (2015) Three further triterpenoid saponins from Gleditsia caspica fruits and protective effect of the total saponin fraction on cyclophosphamide-induced genotoxicity in mice. Z Naturforsch C 70(1-2):31-37. https://doi.org/10.1515/znc-2014-4132
Melek FR, Miyase T, Ghaly NS, Nabil M (2007) Triterpinoid saponins with N-acetyl sugar from the bark of Albizia procera. Phyochemisry 68: 1261-1266. https://doi.org/10.1016/j.phytochem.2007.02.023
Migahid AM (1989) Flora of Saudi Arabia. 3rd ed. King Saud University Press, KSA P 84-87.
Min K, Ebeler SE (2009) Quercetin inhibits hydrogen peroxide-induced DNA damage and enhances DNA repair in Caco-2 cells. Food Chemistry and Toxicology 47(11):2716-2722. https://doi.org/10.1016/j.fct.2009.07.033
Miyase T, Melek FR, Ghaly NS, Warashina T, El-Kady M, Nabil M (2010) Echinocystic acid 3, 16-O-bisglycosides from Albizia procera. Phytochemistry 71: 1375-1380. https://doi.org/10.1016/j.phytochem.2010.05.004
Mohamed HM, Aly MS (2018) Evaluation of genotoxicity of Euphorbia triaculeata Forssk extract on mice bone marrow cells in vivo. Toxicology Reports 5:625–631. https://doi.org/10.1016/j.toxrep.2018.05.007
Orhan F, Gulluce M, Ozkan H, Alpsoy L (2013) Determination of the antigenotoxic potencies of some luteolin derivatives by using a eukaryotic cell system, Saccharomyces cerevisiae. Food Chemistry 141(1): 366-372. https://doi.org/10.1016/j.foodchem.2013.02.089
Rao BSN (2002) Pulses and Legumes as functional foods. Bulletin of Nutrition Foundation of India (NFI) 23(1):14 https://pdfs.semanticscholar.org/864d/f954d57dfe9da77749a95b6b7e5e7ab78866.pdf
Rocha RS, Kassuya CA, Formagio AS, Mauro Mde O, Andrade-Silva M, Monreal AC, Cunha-Laura AL, Vieira Mdo C, Oliveira RJ (2016) Analysis of the anti-inflammatory and chemopreventive potential and description of the antimutagenic mode of action of the Annona crassiflora methanolic extract. Pharmaceutical Biology 54(1):35-47. https://doi.org/10.3109/13880209.2015.1014567
Sanjay K (2003) Saponins of Albizia lebbeck in Alzheimer’s and Parkinson’s diseases. Indian Journal of Natural Product 19(1):42–48
Scarpato R, Bertoli A, Naccarati A, Migliore L, Cocchi L, Barale R, Pistelli L (1998) Different effects of newly isolated saponins on the mutagenicity and cytotoxicity of the anticancer drugs mitomycin C and bleomycin in human lymphocytes. Mutation Research 420 (1-3):49-54. https://doi.org/10.1016/S1383-5718(98)00146-6
Seelinger G, Merfort I, Wölfle U, Schempp CM (2008) Anticarcinogenic effects of the flavonoid luteolin. Molecules 13:2628-2651 https://doi.org/10.3390/molecules13102628
Sharma V, Agrawal RC (2015) Evaluation of anticlastogenic effects of Glycyrrhiza glabra root extract against cyclophosphamide induced chromosomal aberration in Swiss albino mice. J Appl Pharm Sci 5(06):127–132. https://doi.org/10.7324/JAPS.2015.50621
Tripathi DN, Jena GB (2008) Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 248(2-3): 96-103. https://doi.org/10.1016/j.tox.2008.03.015
Vredenburg G, den Braver-Sewradj S, van Vugt-Lussenburg BM, Vermeulen NP, Commandeur JN, Vos JC (2014) Activation of the anticancer drugs cyclophosphamide and ifosfamide by cytochrome P450 BM3 mutants. Toxicology Letter 232(1):182-192. https://doi.org/10.1016/j.toxlet.2014.11.005
Wang Y , Yan T, Ma L, Liu B (2015) Effects of the total saponins from Dioscorea nipponica on immunoregulation in aplastic anemia mice. The American Journal of Chinese Medicine 43(2):289-303. https://doi.org/10.1142/S0192415X15500196
Xiong J, Wang K, Yuan C, Xing R, Ni J, Hu G, Chen F, Wang X (2017) Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med 39(1):113-125. https://doi.org/10.3892/ijmm.2016.2809
Yu J, Chen Y, Zhai L, Zhang L, Xu Y, Wang S, Hu S (2015) Antioxidative effect of ginseng stem-leaf saponins on oxidative stress induced by cyclophosphamide in chickens. Poultry Science 94(5):927-933.https://doi.org/10.3382/ps/pev055
Zaki EL, Springate JE, Taub M (2003) Comparative toxicity of ifosfamide metabolites and protective effect of mesna and amifostine in cultured renal tubule cells. Toxicol In Vitro 17(4): 397–402. https://doi.org/10.1016/S0887-2333(03)00044-4
Zheng L, Zheng J, Wu LJ, Zhao YY (2006) Julibroside J8-induced HeLa cell apoptosis through caspase pathway. J Asian Nat Prod Res 8:457–465. https://doi.org/10.1080/10286020500173309
Zou K, Tong WY, Liang H, Cui, JR, Tu GZ, Zhao YY, Zhang RY (2005) Diastereo isomeric saponins from Albizia julibrissin. Carbohydr Res 340:1329–1334. https://doi.org/10.1016/j.carres.2004.10.027
Acknowledgements
The authors would thank the National Research Centre (NRC, Egypt) for funding this work (10090013).
Funding
This research was financed by the National Research Centre, Egypt, Project No. 10090013.
Author information
Authors and Affiliations
Contributions
MN: collection of plant material, extraction of plants under investigation, and fractionation using different solvents. EEH: participation in practical work, revision of the manuscript, discussion, and revision. NSG and FRM: extraction of plants under investigation and fractionation using different solvents. FAA: practical work and collection of scientific materials. ZMH practical work, revision of the manuscript, and statistical evaluation. MAF and AAF: participation in practical work, participation in the discussion of the manuscript, revision of the manuscript, and statistical evaluation. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective study was reviewed and approved by the Animal Ethics Committee of the National Research Centre, Cairo, Egypt, and was carried out according to the National Institute of Health Guide (NIH) for the Care and Use of Laboratory Animals Guidelines (approval number:1.6.2.1.0).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nabil, M., Hassan, E.E., Ghaly, N.S. et al. Albizia chinensis bark extract ameliorates the genotoxic effect of cyclophosphamide. Bull Natl Res Cent 44, 165 (2020). https://doi.org/10.1186/s42269-020-00422-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-020-00422-9