Abstract
Background
Cutaneous leishmaniasis (CL) is an endemic disease in Bolivia, particularly in the rainforest of Cochabamba, in the municipality of Villa Tunari. The precarious, dispersed, and poorly accessible settlements in these farming communities make it difficult to study them, and there are no epidemiological studies in the area. The aim of the present study was to identify the risk factors associated with cutaneous leishmaniasis.
Methods
A cross-sectional study was conducted in August 2015 and August 2016 in two communities of Villa Tunari, Cochabamba. The cases were diagnosed through clinical examinations, identification of the parasite by microscopic examination, and the Montenegro skin test. Risk factors were identified through logistic regression.
Results
A total of 274 participants (40.9% female and 59.1% male) were surveyed, of which 43% were CL positive. Sex was the only factor associated with CL with three times more risk for men than for women; this finding suggests a sylvatic mechanism of transmission in the area.
Conclusions
It is advisable to focus on education and prevention policies at an early age for activities related to either leisure or work. Further research is needed to assess the influence of gender-associated behavior for the risk of cutaneous leishmaniasis.
Similar content being viewed by others
Background
American cutaneous leishmaniasis (ACL) is an infectious disease produced by the parasite Leishmania spp. This is transmitted by the bite of sandflies that carry the parasite from reservoirs to humans. Reservoirs for the parasite are mostly rodents and large wild mammals. Humans are accidental hosts when they invade the reservoirs and vectors’ ecosystem [1]. In humans, ACL is characterized by chronic skin ulcers that can take from months to years to heal [2].
In 2008, the estimated annual incidence of cutaneous leishmaniasis (CL) in America reached up to 307,800 new cases [3] and it is estimated that 39 million of people are at risk in 21 countries from the Caribbean to South America [4]. The Amazon rainforest is an especially risky area because it is the habitat of the reservoirs and vectors of the Leishmania parasite. This region includes nine nations, and Bolivia is the third country with the most amount of forest, which makes up 70% of its territory. National reports estimate 2300 new cases every year; however, this could be higher due to under-reporting [5]. Incidence rates per 100,000 inhabitants have increased four times during the past 35 years from 4.8 in 1983 to 18.5 in 2012 [6]. This situation can be explained by the accelerated process of deforestation, migration, and colonization of the Bolivian forests for agriculture and for the precariousness and poverty of the settlements. Although these social phenomena allow us to explain the populations’ risk, they do not explain the characteristics at the individual level that define the risk to these exposed populations of becoming ill.
Over the past two decades, several studies have addressed the question of risk factors for ACL. One frequent factor is the human settlements close to a primary forest. When the ecological environments are disturbed, humans are more likely to be exposed to reservoirs and vectors increasing the risk for ACL [7]. Other common factors include gender, age, and outdoor activities. There is a pattern that in sylvatic contexts, men of working age are usually more exposed because of their activities in agriculture and forestry [8,9,10,11,12,13].
Another group of factors are related to housing conditions. Studies have shown that when walls, roofs, and floors are not made of durable materials, cracks can be formed, becoming a shelter or a gateway for vectors into the households [11]. Some studies have also found that using wood as a cooking fuel was a risk factor arguing that it would increase exposure when it is used in open environments [11]. Other studies have however identified this as protective arguing that probably the smoke drives away the vectors [9].
Indoor electricity use has also produced contradictory results. In a study carried out in Ecuador, this turned out to be protective and the authors proposed that light attracts vectors and distances them from people [9]. However, other studies have not confirmed these findings [13].
Another contradictory factor is the presence of domestic animals such as dogs, pigs, or chickens. Some studies have found a protective role in their presence arguing that animals were a preferred source of blood for vectors [10, 14]; however, other studies have suggested that animals could attract the vectors closer to humans, thus becoming an important risk factor [13, 15, 16].
The only study in Bolivia about risk factors was carried out 20 years ago, and the main risk factors reported were occupation, age, and migration [8]; however, this study has only focused on well-known risk factors involved in sylvatic chains of transmission. The constant process of migration, deforestation, and colonization of the forest for agriculture creates the conditions needed to change the leishmaniasis transmission profile from an occupational disease in the forest to a domestic disease in the communities [17]. The purpose of this paper was to assess sociodemographic and housing condition factors as risk factors for CL in a rural area of the Bolivian Amazon.
Methods
Study area
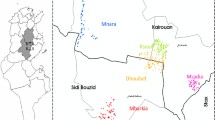
The study was conducted in the municipality of Villa Tunari located in the tropical forest of Cochabamba department (Map 1). In addition, this municipality contains one of the highest ecological reserves in the country, the Isiboro Secure National Park and Indigenous Territory (TIPNIS).
The population registered during the national census in 2012 was 73,914 inhabitants. Migrants represent around 44% of the population, mostly coming from the occidental highlands. The colonization of the tropical forest for agricultural activities is an ongoing process which has been highly criticized by its demographically and environmentally destructive nature [18]. From 1992 to 2012, the population increased by 52%, and today, more than 400 communities are registered in the municipality, 90 of them located in the TIPNIS. These migration movements have also had an impact on the epidemiological profile of leishmaniasis. More than 60% of CL cases in the tropical zone of Cochabamba come from this area, making it one of the municipalities with the highest risk of CL in the country [6].
These human settlements are commonly created in poverty-stricken conditions. The National Census in 2012 reported that nearly all the population in Villa Tunari municipality lived below the poverty line (95%), and many in extreme poverty (22%) in terms of unsatisfactory basic living conditions [19]. Water supply depends on rivers or ponds for most of the households (81%), and firewood is the main cooking fuel in the houses (62%). More than half of the households do not have electricity (56%), and only a few have a sewer system (12%).
Villa Tunari municipality is organized in towns and villages. The primary forest is mainly located in the TIPNIS while outside of the national reserve is mostly secondary forest. Two villages were selected for this study representing different levels of urbanization and vector exposure. Ichoa is located close to the primary forest inside the TIPNIS, and Chipiriri is located outside the reserve representing the secondary forest.
Data collection
A cross-sectional study was conducted during visits to the communities of Ichoa in August 2015 and Chipiriri in August 2016 spending 6 days in each community. The population living in these communities was invited to participate in a CL and ML screening-based study. Multiple invitations were made by their local authorities, by the medical staff of the health center, and by the researchers during their community meetings. The strategy of searching cases trough medical campaigns was chosen instead of house-to-house visits because it was not feasible to find people in their houses. People used to be working on their farms during the day far from their households. In addition, the sylvatic geography where they are settled as well as the scattered distribution of the households made it complicated to find the participants at night.
Medical campaigns consisted of comprehensive physical examination of skin and mucous membranes focused on the detection of present or past cutaneous leishmaniasis conducted by a group of senior researchers from the Center of Tropical Medicine of San Simon University in Cochabamba. Whenever a skin ulcer was present in the participants, a sample was taken for direct microscopic examination (DME).
The criteria used to identify CL infections were (1) presence of skin ulcers on which Leishmania parasite was identified through DME (sensitivity of 50–68%) [20, 21] and (2) skin scars with history of skin ulcers that lasted more than 2 weeks before healing and who would had a positive reaction to the Montenegro skin test (MST). This test has been successfully used in several epidemiological studies to assess exposition to the Leishmania parasite and leishmaniasis infection [22,23,24,25,26,27]. The MST sensitivity and specificity to detect parasite exposure were reported at over 97 and 93%, respectively, which improves with increased exposure time [28,29,30]. The reaction to MST was measured after 48 h by the ballpoint pen method; if induration was 5 mm or larger, the test was considered positive. The antigens used for the MST test were produced by the Institute of Tropical Medicine at the Cayetano Heredia University, Peru.
A questionnaire was used to collect data regarding house construction materials (wall and roof), basic services (electricity, gas, drinking water networks, and sewer system), and sociodemographic characteristics (age, gender, farming activity, schooling level, and mother tongue) which was adapted from the Demography and Health National Survey [31]. Finally, information related to forest exposure and use of protective measures against mosquito bites such as the use of bed nets, use of repellants, and household fumigations were collected from all the participants.
Statistical analysis
The descriptive statistics were expressed as percentages and as a mean and standard deviation (SD) for categorical and continuous variables, respectively. Logistic regression was used to test the association between the potential risk factors and the outcome. A univariate analysis was performed with all the variables included in the study, and those with a p value less than 0.2 were included in the multivariate analysis. There was no collinearity among the variables included in the multivariate analysis assessed with the variance inflation factor. All analyses were carried out using the SPSS software.
Results
The sample consisted of 274 subjects from Ichoa community (n = 200) and Chipiriri community (n = 74). Participants were mostly young people (median age 35 years old). Work activities were mostly agriculture for men (79.6%) and housework for women (78.6%). Migrant people from the highlands represented more than half of the sample (56.6%), and their mother tongue was mostly Quechua (82.8%). The educational level was 6.2 years on average. Of the total sample, 119 participants (43.4%) had CL, and of them, 116 cases (97.5%) corresponded to past infections with skin scars and history of chronic infection that were positive for MST, while 3 cases (2.5%) corresponded to active skin ulcers that were positive for DME. The majority of CL cases were men (69.7%), and the median age was 35 years old (range 14–73). Most cases were from the Ichoa community (79.8%) compared to the Chipiriri community (20.2%). A greater proportion of cases were migrants from the highlands (58.0%), with Quechua and Aymara as their mother tongue (87.4%) and with a residence time in the communities greater than 10 years (54.2%). More than half of the cases worked in agriculture (64.7%), and although the majority of the cases could read (89.0%), the schooling level was low (6.2 years of education in average) (Table 1).
Regarding the housing conditions of the sample, wall materials were mostly wood or plastered mud (70.7%), the drainage system was mostly cesspool or none (81.7%), and the fuel used for cooking was primarily firewood (77.6%). Houses were mostly small having fewer than three bedrooms (64.2%), and the human density was 2.7 people per bedroom on average. Most of the participants raised hens (86.5%), while less than half owned dogs (40.4%). Housing conditions of cases showed some interesting findings. There was a greater proportion of cases with wood (78.2%) compared to concrete (21.8%) as the material of the walls, with cesspool or none (88.2%) as the drainage system, and wood (81.4%) instead of gas (18.6%) as the cooking fuel. Additionally, the majority of cases lived in households with less than three rooms (71.4%). Finally, there were more cases who had hens (92.0%) but less of those who owned dogs (38.6%) (Table 2).
Regarding the use of protective measures of the sample, the routine use of repellent was rare (2.9%), and the majority described having sprayed the house sometimes or very often (79.5%). Almost all the participants used a bed net to sleep (90.5%). Most participants described sleeping with open windows (68.6%). Among CL cases, almost all referred to always use bed nets (92.4%); however, most of them always slept with the windows open (66.7%). The majority of the cases fumigated their household sometimes (54.2%) or always (23.7%) and never used repellents (78.0%) (Table 3).
In the univariate analysis, the sociodemographic factors associated with an increased risk of CL were to be male, a farmer, and living in a village close to the primary forest. Some housing conditions such as wall materials, sewage system, number of rooms in the house or number of persons per room, and ownership of chicken were also significant. In the multivariate analysis, sex remained as the only one statistically significant risk factor where men had three times increased odds of being infected with CL compared to women (Table 4).
Discussion
This is the first study to assess both sociodemographic characteristics and housing conditions as risk factors for leishmaniasis in Bolivia. In our study, sex was the only one statistically significant variable for CL risk. This result is in agreement with the findings of the previous study of the risk factors carried out in 1997, where only sex and age were significant. These findings reflect of a mostly sylvatic transmission of CL in Bolivia where the absence of significance with the sociodemographic and housing condition variables could be interpreted as low peridomestic transmission for CL in the region. The importance of the variable sex is consistent when studies have been performed in areas where the chain transmission is sylvatic [15, 32, 33]. However, in research carried out in areas where transmission is peridomestic, no significant difference by sex has been found [34, 35].
Differences in risk for CL between men and women are related to differences in gender roles and not to biological characteristics associated with sex [36]. In fact, in sylvatic environments, leishmaniasis disease can be considered as a lifestyle bodymark [37] of men who have higher exposition to the transmitter vector of leishmaniasis. In these environments, the ideal male figure is characterized by being strong, courageous, a risk-taker, and able to withstand extensive physical sacrifices as well as dealing with danger [38]. For this reason, from a very young age, males are involved in recreational activities performed in the forest, such as hunting and fishing. One study conducted in Brazil showed that leisure activities in the forest in addition to fishing and hunting represented a higher risk of CL [13]. In addition, when males reach adulthood, their work activities—such as agriculture, gold mining, logging, and forestry—are also carried out inside the forest. One study performed in Ecuador supports this idea showing that the risk of CL was similar during adolescence but increased for men sharply into adulthood when they started work activities in the rainforest [9].
Regarding housing conditions, in our study, none of these factors were statistically significantly related to CL. Studies from Argentina and Ecuador have shown that the characteristics of the domicile are important determinants for the risk of becoming ill when the transmission chain is peridomestic [9, 11]. In this sense, it is possible to characterize vulnerable dwellings as those constructed with non-durable materials, which allow the formation of cracks through which the vectors can enter into houses. The lack of relevance of these factors in our study may be supportive of a sylvatic transmission chain.
The presence of animals in the house is also important when the transmission is peridomestic. In our study, the possession of hens was not statistically significant in the adjusted model. While some studies have suggested the certain role of hens in the transmission of leishmaniasis [39, 40], others have questioned the effect of avian blood on Leishmania development in sand flies [41].
No association between possession of dogs and CL risk was found either. The role of dogs in CL transmission is still discussed in the scientific literature. Some studies have suggested that the dog may act as a reservoir because a greater number of human cases were found in areas with high incidence of canine cases [42]. Other studies have shown the presence of parasitic DNA in the blood and bone marrow of these animals. However, these findings are considered to be circumstantial. Experimental studies showed that the vector was not infected when they were fed with blood from non-ulcerated areas of infected dogs. Another study showed that the percentage of infected vectors consuming blood from an infected dog was less than 1% [43]. Additionally, a review study on the possible role of a dog as a reservoir showed that there was no conclusive information to confirm this assumption after reviewing more than 90 studies [44].
Finally, the use of bed nets was not important in our study probably because most of the participants affirmed using them frequently to sleep. Studies from Colombia and Venezuela have shown that the use of bed nets only is not protective and that impregnation with insecticides such as deltamethrin [45] or lambdacyhalothrin [46] is important to reduce the risk of being bitten by the vector. Their utility seems to be very promising because of the long residual effect, with a residual lethal effect close to 100% after 12 months [47].
Limitations
This study has three limitations related to the study design, the sample size, and the selection bias.
First, the cross-sectional study design does not allow us to discern causal relationships; however, this study does not aim to identify causal relations, but rather using statistical multivariate methods, it aims to test the effects of some new variables (sociodemographic and housing condition factors) as risk factors in the presence of other well-known risk factors such as sex, age, and occupation. Second, the small sample size is probably the most important limitation of the study. This limitation could be responsible for the lack of statistical significance of the variables included in the multivariate model. This limitation was mainly due to logistical difficulties related to the characteristics of the disease. On the one hand, CL occurs mainly in populations settled in sylvatic areas. Their communities are mostly small, scattered, and difficult to reach. On the other hand, their agricultural activities limited the participation of some of the inhabitants, since it was not possible to locate them in their houses during the day.
Third, given that the study did not use a random sampling method, a selection bias influenced by the willingness to participate in the study could have been possible. However, with the available data, the extent of this bias could not be assessed. Some variables such as the source of water, electricity, and wall material did not have enough variation in one of the communities which may have affected the analysis estimation.
Conclusions
This is the first study performed in Bolivia on risk factors that consider sociodemographic and housing conditions in which sex was the only factor associated with CL. This finding suggests a sylvatic transmission mechanism in the study area. However, it is necessary to carry out larger studies in different locations of the country that will allow us to identify whether different mechanisms of transmission are present and in consequence whether the population is at risk. In addition, it is advisable to focus on CL education and prevention policies at an early age in sylvatic areas, with the aim of encouraging the use of protective measures that reduce vector exposure when people enter into the forest, for activities related to either leisure or work. This study contributes to the construction of knowledge on a little explored issue such as the risk factors in the sylvatic areas of the American Amazon.
Change history
21 June 2018
In the original publication of this article [1], the authors noted that some data were incorrect in Table 4.
Abbreviations
- ACL:
-
American cutaneous leishmaniasis
- CL:
-
Cutaneous leishmaniasis
- MST:
-
Montenegro skin test
- TIPNIS:
-
Isiboro Secure National Park and Indigenous Territory
References
Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–18.
Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010(949):xii-xiii, 1–186, back cover.
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671.
Ashford RW, Desjeux P, deRaadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8(3):104–5.
National Program of Leishmaniasis Control, Ministry of Health of Bolivia. National Manual and Guidelines for technical procedures in Leishmaniasis. La Paz 2012.
National Program of Leishmaniasis Control, Ministry of Health of Bolivia. National Manual and Guidelines for technical procedures in Leishmaniasis. La Paz 2015.
Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95(3):239–43.
Alcais A, Abel L, David C, Torrez ME, Flandre P, Dedet JP. Risk factors for onset of cutaneous and mucocutaneous leishmaniasis in Bolivia. Am J Trop Med Hyg. 1997;57(1):79–84.
Armijos RX, Weigel MM, Izurieta R, Racines J, Zurita C, Herrera W, et al. The epidemiology of cutaneous leishmaniasis in subtropical Ecuador. Tropical Med Int Health. 1997;2(2):140–52.
Davies CR, Llanos-Cuentas EA, Campos P, Monge J, Villaseca P, Dye C. Cutaneous leishmaniasis in the Peruvian Andes: risk factors identified from a village cohort study. Am J Trop Med Hyg. 1997;56(1):85–95.
Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg. 2003;68(5):519–26.
Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis. 1993;168(3):709–14.
Pedrosa Fde A, Ximenes RA. Sociodemographic and environmental risk factors for American cutaneous leishmaniasis (ACL) in the State of Alagoas, Brazil. Am J Trop Med Hyg. 2009;81(2):195–201.
Ranasinghe S, Wickremasinghe R, Munasinghe A, Hulangamuwa S, Sivanantharajah S, Seneviratne K, et al. Cross-sectional study to assess risk factors for leishmaniasis in an endemic region in Sri Lanka. Am J Trop Med Hyg. 2013;89(4):742–9.
Araujo AR, Portela NC, Feitosa AP, Silva OA, Ximenes RA, Alves LC, et al. Risk factors associated with American cutaneous leishmaniasis in an endemic area of Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:86.
Votýpka J, Kasap OE, Volf P, Kodym P, Alten B. Risk factors for cutaneous leishmaniasis in Cukurova region, Turkey. Trans R Soc Trop Med Hyg. 2012;106(3):186–90.
Oryan A, Akbari M. Worldwide risk factors in leishmaniasis. Asian Pac J Trop Med. 2016;9(10):925–32.
de la Cuadra F. Indigenous people, socio-environmental conflict and post-development in Latin America. Ambiente Sociedade. 2015;18:23–40.
National Institute of Statistics. Population and Housing Census 2012. (2015, Jan 15th). Statistics summary of the municipality of Villa Tunari, Department of Cochabamba 2012. Available from: https://www.ine.gob.bo/. Accessed 10 Apr 2017.
Ertabaklar H, Ozlem Caliskan S, Boduc E, Ertug S. Comparison of direct microscopy, culture and polymerase chain reaction methods for the diagnosis of cutaneous leishmaniasis. Mikrobiyol Bul. 2015;49(1):77–84.
Pourmohammadi B, Motazedian MH, Hatam GR, Kalantari M, Habibi P, Sarkari B. Comparison of three methods for diagnosis of cutaneous leishmaniasis. Iran J Parasitol. 2010;5(4):1–8.
Ampuero J, Urdaneta M, Macedo Vde O. Risk factors for cutaneous leishmaniasis transmission in children aged 0 to 5 years in an endemic area of Leishmania (Viannia) braziliensis. Cad Saude Publica. 2005;21(1):161–70.
Bettaieb J, Toumi A, Chlif S, Chelghaf B, Boukthir A, Gharbi A, et al. Prevalence and determinants of Leishmania major infection in emerging and old foci in Tunisia. Parasit Vectors. 2014;7:386.
de Oliveira-Neto MP, Mattos MS, Perez MA, Da-Cruz AM, Fernandes O, Moreira J, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol. 2000;39(7):506–14.
Oliveira F, Doumbia S, Anderson JM, Faye O, Diarra SS, Traore P, et al. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on Leishmanin skin test surveys. PLoS Negl Trop Dis. 2009;3(12):e565.
Silveira FT, Lainson R, Pereira EA, de Souza AA, Campos MB, Chagas EJ, et al. A longitudinal study on the transmission dynamics of human Leishmania (Leishmania) infantum chagasi infection in Amazonian Brazil, with special reference to its prevalence and incidence. Parasitol Res. 2009;104(3):559–67.
Sordo L, Gadisa E, Custodio E, Cruz I, Simon F, Abraham Z, et al. Low prevalence of Leishmania infection in post-epidemic areas of Libo Kemkem, Ethiopia. Am J Trop Med Hyg. 2012;86(6):955–8.
Hashemi SN, Mohebali M, Mansouri P, Bairami A, Hajjaran H, Akhoundi B, et al. Comparison of leishmanin skin test and direct smear for the diagnosis of cutaneous leishmaniasis. Acta Med Iran. 2011;49(3):136–41.
Marques MJ, Volpini AC, Machado-Coelho GL, Machado-Pinto J, da Costa CA, Mayrink W, et al. Comparison of polymerase chain reaction with other laboratory methods for the diagnosis of American cutaneous leishmaniasis: diagnosis of cutaneous leishmaniasis by polymerase chain reaction. Diagn Microbiol Infect Dis. 2006;54(1):37–43.
Skraba CM, de Mello TF, Pedroso RB, Ferreira EC, Demarchi IG, Aristides SM, et al. Evaluation of the reference value for the Montenegro skin test. Rev Soc Bras Med Trop. 2015;48(4):437–44.
Coa R, Ochoa LH. Encuesta nacional de demografía y salud: endsa 2008: Ministerio de Salud y Deportes; 2009.
Guerra JAdO, Maciel MG, Guerra MVdF, Talhari AC, Prestes SR, Fernandes MA, et al. Tegumentary leishmaniasis in the state of Amazonas: what have we learned and what do we need? Rev Soc Bras Med Trop 2015;48:12–19.
Martins LM, Rebêlo JMM, Santos MCFVd, Costa JML, Silva ARd, Ferreira LA. Eco-epidemiology of cutaneous leishmaniasis in Buriticupu, Amazon region of Maranhao state, Brazil, 1996–1998. Cadernos de saude publica 2004;20(3):735–743.
Velez ID, Hendrickx E, Robledo SM, del Pilar Agudelo S. Gender and cutaneous leishmaniasis in Colombia. Cad Saude Publica. 2001;17(1):171–80.
Monteiro WM, Neitzke-Abreu HC, Ferreira ME, Melo GC, Barbosa M, Lonardoni MV, et al. Population mobility and production of American tegumentary leishmaniasis in the state of Parana, southern Brazil. Rev Soc Bras Med Trop. 2009;42(5):509–14.
Krieger N. Genders, sexes, and health: what are the connections—and why does it matter? Int J Epidemiol. 2003;32(4):652–7.
Krieger N. Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59(5):350–5.
Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50(10):1385–401.
Oliveira-Pereira YN, Moraes JLP, Lorosa ES, Rebêlo JMM. Feeding preference of sand flies in the Amazon, Maranhão state, Brazil. Cadernos de Saúde Pública. 2008;24(9):2183–6.
Fonteles RS, Azevêdo PCB, Vasconcelos GC, Rebêlo JMM, Kuppinger O, Lorosa ES, et al. Preferência alimentar sanguínea de Lutzomyia whitmani (Diptera, Psychodidae) em área de transmissão de leishmaniose cutânea americana, no Estado do Maranhão, Brasil. Rev Soc Bras Med Trop. 2009:647–50.
Pruzinova K, Votypka J, Volf P. The effect of avian blood on Leishmania development in Phlebotomus duboscqi. Parasit Vectors. 2013;6(1):254.
Aguilar CM, Rangel EF, Garcia L, Fernandez E, Momen H, Grimaldi Filho G, et al. Zoonotic cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis associated with domestic animals in Venezuela and Brazil. Mem Inst Oswaldo Cruz. 1989;84(1):19–28.
Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149(3–4):139–46.
Reithinger R, Davies CR. Is the domestic dog (Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am J Trop Med Hyg. 1999;61(4):530–41.
Alexander B, Usma MC, Cadena H, Quesada BL, Solarte Y, Roa W, et al. Evaluation of deltamethrin-impregnated bednets and curtains against phlebotomine sandflies in Valle del Cauca, Colombia. Med Vet Entomol. 1995;9(3):279–83.
Kroeger A, Avila EV, Morison L. Insecticide impregnated curtains to control domestic transmission of cutaneous leishmaniasis in Venezuela: cluster randomised trial. BMJ. 2002;325(7368):810–3.
Bray DP, Hamilton JG. Insecticide-impregnated netting as a potential tool for long-lasting control of the leishmaniasis vector Lutzomyia longipalpis in animal shelters. Parasit Vectors. 2013;6:133.
Acknowledgements
We are grateful to the participants and the medical staff in the healthcare centers of Chipiriri and Ichoa who collaborated in this study.
Funding
This research and publication was funded by the Swedish International Development Cooperation Agency (SIDA). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are available upon request.
Author information
Authors and Affiliations
Contributions
DE, ER, MGR, DI, and MSS contributed to the conception and design of the study. DE, ER, and MGR participated in the data collection. DE, IG, AKH, and MSS contributed to the drafting of the manuscript. All authors critically revised the manuscript and gave final approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of the study was obtained from the Ethical Committee of the School of Medicine in San Simon University. The research team attended monthly community meetings organized by the board of the Local Farmers Union. The community received information on leishmaniasis disease, as well as the nature and importance of the study. The researchers asked for permission from the population to proceed with the study. Individual written consent was signed by each participant and for children under 15 years old, written consent was also sought from their parents. No incentives were provided to participants.
Participants with parasitological confirmation of active CL were assisted with diagnosis and treatment free of charge. Participants with skin scars due to past chronic skin ulcers that resulted positive to MST were assessed looking for signs of reactivation of CL and mucosal complications. Given that none of the participants showed these problems, they were advised to contact the researchers in case any sign or symptom suggestive of these problems would appear.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Eid, D., Guzman-Rivero, M., Rojas, E. et al. Risk factors for cutaneous leishmaniasis in the rainforest of Bolivia: a cross-sectional study. Trop Med Health 46, 9 (2018). https://doi.org/10.1186/s41182-018-0089-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-018-0089-6