Abstract
Purpose
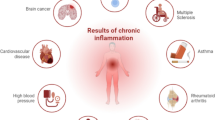
Chronic low-grade inflammation is associated with chronic non-communicable diseases (CNCDs). Bioactive compounds (BC) may influence the reduction of low-grade inflammation. This narrative review highlights the relationship between CNCDs, inflammation, and ingestion of catechins, curcumin, quercetin, and resveratrol.
Methods
A PubMed research was conducted in the last 10 years. The search was for human studies, and we selected “case reports”; “clinical study”; “clinical trial”; “clinical trial, phase I”; “clinical trial, phase II”; “clinical trial, phase III”; “clinical trial, phase IV”; “comparative study”; “controlled clinical trial”; “multicenter study”; “observational study”; and “randomized clinical trial”. The research was carried out individually for each BC of the diet, and we selected studies related to CNCDs and risk factors for cardiometabolic diseases. The keywords used were “catechin,” “curcumin,” “quercetin,” and “resveratrol”, in addition to “inflammation.”
Results
There are no recommendations for BC intake. The studies in this review analyzed different doses, forms of presentation, clinical conditions, and periods of supplementation. Most articles on catechin, curcumin, and quercetin had a clear beneficial relationship between inflammation, CNCDs, and risk factors for cardiometabolic diseases. On the other hand, studies on resveratrol are inconclusive.
Conclusion
Some studies have shown beneficial effects of BC; however, the studies are not conclusive. In different situations, BC have no significant effects, and, at other times, they may even have a detrimental effect. Further studies are needed to establish which dose produces beneficial health effects and which clinical conditions can benefit from the intake of dietary sources from these BC.


Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AH:
-
Arterial hypertension
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BC:
-
Bioactive compounds
- BMI:
-
Body mass index
- CAMs:
-
Cell adhesion molecules
- CNCDs:
-
Chronic non-communicable diseases
- COX- 2:
-
Cyclooxygenase-2
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- EC:
-
Epicatechin
- ECG:
-
Epicatechin gallate
- EGCG:
-
Epigallocatequin gallate
- HbA1c:
-
Glycated hemoglobin
- HDL-c:
-
High-density lipoprotein cholesterol
- HOMA:
-
Homeostasis evaluation model
- IL:
-
Interleukin
- LDL-c:
-
Low-density lipoprotein cholesterol
- MS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- NF-kB:
-
Nuclear factor-kappa B
- RSV:
-
Resveratrol
- T2D:
-
Type 2 diabetes
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor-alpha
References
WHO (2018). Noncommunicable Diseases Report.
Calder PC, Albers R, Antoine J-M, Blum S, Bourdet-Sicard R, Ferns GA, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101:1–45. https://doi.org/10.1017/s0007114509377867.
Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106:S5–S78. https://doi.org/10.1017/S0007114511005460.
Leiherer A, Mündlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vasc Pharmacol. 2013;58:3–20.
Bastos DHM, Rogero MM, Arêas JAG. Mecanismos de ação de compostos bioativos dos alimentos no contexto de processos inflamatórios relacionados à obesidade. Arq Bras Endocrinol Metabol. 2009;53:646–56. https://doi.org/10.1590/s0004-27302009000500017.
Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10(5):604.
Gallot YS, McMillan JD, Xiong G, et al. Distinct roles of TRAF6 and TAK1 in the regulation of adipocyte survival, thermogenesis program, and high-fat diet-induced obesity. Oncotarget. 2017;8(68):112565–83. https://doi.org/10.18632/oncotarget.22575.
Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–9.
Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4):432.
Plenchette S, Romagny S, Laurens V, Bettaieb A. S-nitrosylation in TNF superfamily signaling pathway: implication in cancer. Redox Biol. 2015;6:507–15.
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230–42. https://doi.org/10.1093/ajcn/81.1.230s.
Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. https://doi.org/10.1016/j.copbio.2008.03.003.
De Oliveira DM, Bastos DHM. Biodisponibilidade de Ácidos fenólicos. Quim Nova. 2011;34:1051–6. https://doi.org/10.1590/S0100-40422011000600023.
Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–85S. https://doi.org/10.1093/jn/134.12.3479S.
Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev Med (Baltim). 2012;54.Suppl(Suppl):S29–3.
Rescigno T, Tecce MF, Capasso A. Protective and restorative effects of nutrients and phytochemicals. Open Biochem J. 2018;12:46–64. https://doi.org/10.2174/1874091X01812010046.
Siriwardhana N, Kalupahana NS, Cekanova M, LeMieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24:613–23.
Fan FY, Sang LX, Jiang M, McPhee DJ. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22(3):484.
He Y, Yue Y, Zheng X, Zhang K, Chen S, du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–213.
Quintanilha BJ, Reis BZ, Silva Duarte GB, et al. Nutrimiromics: role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients. 2017;9(11):1168.
Cialdella-Kam L, Ghosh S, Meaney MP, et al. Quercetin and green tea extract supplementation downregulates genes related to tissue inflammatory responses to a 12-week high fat-diet in mice. Nutrients. 2017;9(7):773. https://doi.org/10.3390/nu9070773.
Rosa FT, Zulet MÁ, Marchini JS, Martínez JA. Bioactive compounds with effects on inflammation markers in humans. Int J Food Sci Nutr. 2012;63:749–65. https://doi.org/10.3109/09637486.2011.649250.
Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev. 2018;31:85–97. https://doi.org/10.1017/s095442241700021x.
Cai ZY, Li XM, Liang JP, Xiang LP, Wang KR, Shi YL, et al. Bioavailability of tea catechins and its improvement. Molecules. 2018;23:10–3. https://doi.org/10.3390/molecules23092346.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. https://doi.org/10.1021/mp700113r.
Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572–82. https://doi.org/10.2174/09298673113209990120.
Wenzel E, Somoza V. Review metabolism and bioavailability of trans-resveratrol. 2005;472–481. https://doi.org/10.1002/mnfr.200500010.
Walle T. Bioavailability of resveratrol. 2011;1215:9–15. https://doi.org/10.1111/j.1749-6632.2010.05842.x.
Chimento A, De Amicis F, Sirianni R, et al. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int J Mol Sci. 2019;20(6):1381.
Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med Cell Longev. 2015;2015:1–13. https://doi.org/10.1155/2015/837042.
Basu A, Du M, Sanchez K, et al. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011;27:206–13. https://doi.org/10.1016/j.nut.2010.01.015.
Rondanelli M, Opizzi A, Perna S, Faliva M, Solerte SB, Fioravanti M, et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine. 2013;44:391–401. https://doi.org/10.1007/s12020-012-9863-0.
Sakata R, Nakamura T, Torimura T, et al. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int J Mol Med. 2013;32:989–94. https://doi.org/10.3892/ijmm.2013.1503.
Gutiérrez-Salmeán G, Ortiz-Vilchis P, Vacaseydel CM, Rubio-Gayosso I, Meaney E, Villarreal F, et al. Acute effects of an oral supplement of (−)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct. 2014;5:521–7. https://doi.org/10.1039/c3fo60416k.
Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:1459–63. https://doi.org/10.3945/jn.115.211888.
Gutiérrez-Salmeán G, Meaney E, Lanaspa MA, Cicerchi C, Johnson RJ, Dugar S, et al. A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: unique in vitro effects. Int J Cardiol. 2016;223:500–6. https://doi.org/10.1016/j.ijcard.2016.08.158.
Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25:144–50. https://doi.org/10.1016/j.jnutbio.2013.09.013.
Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, et al. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci World J. 2014;2014:1–6. https://doi.org/10.1155/2014/898361.
Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015;34:1101–8. https://doi.org/10.1016/j.clnu.2014.12.019.
Rahmani S, Asgary S, Askari G, et al. Treatment of non-alcoholic fatty liver disease with Curcumin: a randomized placebo-controlled trial. Phyther Res. 2016;30(9):1540–8. https://doi.org/10.1002/ptr.5659.
Kocher A, Bohnert L, Schiborr C, Frank J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol Nutr Food Res. 2016;60:1555–63. https://doi.org/10.1002/mnfr.201501034.
Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578–82. https://doi.org/10.1016/j.biopha.2016.05.037.
Panahi Y, Hosseini MS, Khalili N, Naimi E, Soflaei SS, Majeed M, et al. Effects of supplementation with curcumin on serum adipokine concentrations: a randomized controlled trial. Nutrition. 2016;32:1116–22. https://doi.org/10.1016/j.nut.2016.03.018.
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía L, Majeed M, et al. Effects of Curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res (Stuttg). 2018;68:403–9. https://doi.org/10.1055/s-0044-101752.
Vors C, Couillard C, Paradis ME, Gigleux I, Marin J, Vohl MC, et al. Supplementation with resveratrol and curcumin does not affect the inflammatory response to a high-fat meal in older adults with abdominal obesity: a randomized, placebo-controlled crossover trial. J Nutr. 2018;148:379–88. https://doi.org/10.1093/jn/nxx072.
Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–74. https://doi.org/10.1017/S0007114509359127.
Egert S, Boesch-Saadatmandi C, Wolffram S, Rimbach G, Müller MJ. Serum lipid and blood pressure responses to Quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr. 2010;140:278–84. https://doi.org/10.3945/jn.109.117655.
Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, et al. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49:345–55. https://doi.org/10.1007/s00394-010-0092-0.
Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–70. https://doi.org/10.1523/JNEUROSCI.0385-14.2014.
Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br J Nutr. 2015;114:1263–77. https://doi.org/10.1017/S0007114515002950.
Cialdella-Kam L, Nieman D, Knab A, Shanely R, Meaney M, Jin F, et al. A mixed flavonoid-fish oil supplement induces immune-enhancing and anti-inflammatory transcriptomic changes in adult obese and overweight women—a randomized controlled trial. Nutrients. 2016;8:277. https://doi.org/10.3390/nu8050277.
Nieman DC, Ramamoorthy S, Kay CD, Goodman CL, Capps CR, Shue ZL, et al. Influence of ingesting a flavonoid-rich supplement on the metabolome and concentration of urine phenolics in overweight/obese women. J Proteome Res. 2017;16:2924–35. https://doi.org/10.1021/acs.jproteome.7b00196.
Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: a randomized double-blinded, placebo-controlled crossover trial. Eur J Nutr. 2017;56:2265–75. https://doi.org/10.1007/s00394-016-1267-0.
Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. https://doi.org/10.1080/21623945.2017.1402151.
Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Salvador J, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;11:1–13. https://doi.org/10.3390/nu11020454.
Bakker GCM, Van Erk MJ, Pellis L, et al. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: a nutrigenomics approach. Am J Clin Nutr. 2010;91:1044–59. https://doi.org/10.3945/ajcn.2009.28822.
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22. https://doi.org/10.1016/j.cmet.2011.10.002.
Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–63. https://doi.org/10.1016/j.amjcard.2012.03.030.
Bo S, Ciccone G, Castiglione A, Gambino R, de Michieli F, Villois P, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–31. https://doi.org/10.2174/0929867311320100009.
Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29:178–83. https://doi.org/10.1016/j.nut.2012.07.006.
Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. https://doi.org/10.1007/s10557-012-6427-8.
Tomé-Carneiro J, Larrosa M, Yáñez-Gascón MJ, Dávalos A, Gil-Zamorano J, Gonzálvez M, et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013;72:69–82. https://doi.org/10.1016/j.phrs.2013.03.011.
Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–43. https://doi.org/10.1016/j.nutres.2014.09.005.
Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O'Moore–Sullivan TM, Lee P, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2092–103. https://doi.org/10.1016/j.cgh.2014.02.024.
Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. 2015;47:226–32. https://doi.org/10.1016/j.dld.2014.11.015.
Van der Made SM, Plat J, Mensink RP. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: a randomized, placebo-controlled crossover trial. PLoS One. 2015;10:e0118393. https://doi.org/10.1371/journal.pone.0118393.
Di Renzo L, Marsella LT, Carraro A, et al. Changes in LDL oxidative status and oxidative and inflammatory gene expression after red wine intake in healthy people: a randomized trial. Mediat Inflamm. 2015;2015:317348–13. https://doi.org/10.1155/2015/317348.
Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;114:796–803. https://doi.org/10.1017/s0007114515002433.
Xue M, Weickert MO, Qureshi S, Kandala NB, Anwar A, Waldron M, et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes. 2016;65:2282–94. https://doi.org/10.2337/db16-0153.
Bo S, Ponzo V, Ciccone G, Evangelista A, Saba F, Goitre I, et al. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol Res. 2016;111:896–905. https://doi.org/10.1016/j.phrs.2016.08.010.
Kjær TN, Ornstrup MJ, Poulsen MM, Stødkilde-Jørgensen H, Jessen N, Jørgensen JOL, et al. No beneficial effects of resveratrol on the metabolic syndrome: a randomized placebo-controlled clinical trial. J Clin Endocrinol Metab. 2017;102:1642–51. https://doi.org/10.1210/jc.2016-2160.
Van der Made SM, Plat J, Mensink RP. Trans-resveratrol supplementation and endothelial function during the fasting and postprandial phase: a randomized placebo-controlled trial in overweight and slightly obese participants. Nutrients. 2017;9:596. https://doi.org/10.3390/nu9060596.
Author information
Authors and Affiliations
Contributions
G.C.C. and M.M.R. were responsible for the study concept. The search of the studies and the initial manuscript preparation were performed by G.C.C. G.C.C. and L.T.Y were responsible for the study analyses and design and wrote the manuscript. The manuscript was revised by L.T.Y., T.A.F.C., and M.M.R. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Rights and permissions
About this article
Cite this article
Corsi, G.C., Yoshime, L.T., Corrêa, T.A.F. et al. Bioactive compounds and inflammation: an overview. Nutrire 45, 14 (2020). https://doi.org/10.1186/s41110-020-00118-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-020-00118-0