Abstract
Background
Probiotic supplementation alters oral microbiota composition and could reduce the risk or treat oral cavity diseases, such as dental caries, which are considered a public health problem.
Aim
To summarize the therapeutic effects of probiotics in caries and to verify whether this intervention is capable of replacing conventional treatment in human beings.
Methods
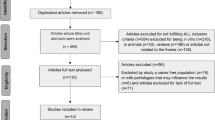
The search of the studies was carried out in the PubMed database in October 2017, without limiting the publication period. The keyword combination used was “Probiotics” and “Dental caries.” Forty-two original articles that evaluated the effect of probiotic supplementation on caries treatment in humans were included in the study.
Results
Most of the studies evaluated bacteria of the genus Lactobacillus. The main therapeutic effects are related to the reduction of the Streptococcus mutans oral count, increased Lactobacillus oral count, and reduction in the incidence of caries. Evidence on the therapeutic effects of the Bifidobacterium and Streptococcus genres is scarce and conflicting, making it difficult to recommend them for use in clinical practice. Only a few studies administered probiotics without conventional treatments, such as fluoride. Although probiotic supplementation presented interesting properties, the therapeutic effects are more pronounced when probiotic and fluoride are applied together.
Conclusion
Probiotics, especially of the Lactobacillus genus, can be used as adjuvants, but cannot replace the conventional treatments of caries.
Similar content being viewed by others
Background
Probiotics are considered “live microorganisms that, when administered in adequate amounts, confer health benefits on the host” [1, 2]. One of the main probiotic health claims is the positive change in microbiota composition [3, 4], traditionally investigated in regard to intestinal health [5,6,7,8,9]. However, there is evidence that probiotic therapy also alters oral microbiota and reduces the risk or could be used in the treatment of oral cavity diseases [10,11,12]. Briefly, the mechanisms proposed for probiotic actions consist of adhesion to the tooth surface and competition with cariogenic bacteria to reduce their growth [13] and the modulation of microbiota composition, thereby promoting oral health [14]. When in high amounts in the oral cavity, beneficial bacteria, such as those belonging to the Lactobacillus genus, inhibit the growth of pathogenic bacteria, such as cariogenic streptococci, preventing the development of dental caries [14, 15]. This is a multifactorial disease caused by the interaction of dietary sugar, dental biofilm, and the host’s dental tissue. Specific microorganisms, such as Streptococcus mutans, produce acid after consuming sugar, and this leads to the demineralization of dental enamel [16].
Dental caries is one of the most common diseases worldwide [17], being considered as a public health problem in children, culminating in excessive costs [18]. In this sense, interventions, highlighting probiotic supplementation, have been proposed in order to reduce the incidence of this disease. Recent studies indicate that probiotic supplementation reduces the risk of caries [17, 19], since they may play a role as antagonist agent on Streptococcus mutans and other acidogenic and aciduric microorganisms [17], being linked not only to the incidence reduction but also to the treatment of this disease [14, 18], although the literature concerning the effects of these microorganisms is still conflicting [20]. Nonetheless, whether probiotic supplementation is more efficient to replace conventional caries treatment remains to be elucidated. Thus, this review aimed at appraises trials assessing probiotics for managing caries and examine whether this intervention can replace conventional treatment in humans.
Methods
Eligibility criteria
In the present review, we only included trials assessing the in vivo role of probiotic supplementation on caries (incidence, risk factor, etc.) in subjects without any stated medical condition and published in the English language.
Search strategy
The search of the studies was carried out using the PubMed database in October 2017, without limiting the publication period. Keywords were selected from Mesh (Medical Subject Headings) and combined as “Probiotics” AND “Dental caries.”
A total of 165 articles was found, including experimental studies with animals (n = 4), in vitro (n = 41), in situ (n = 1), review articles (n = 64), and human studies (n = 55). Regarding the human trials, 18 studies did not meet the inclusion criteria and two articles did not provide the complete version, resulting in 35 original articles included in this review. We also included seven trials that were not found in the search but were cited in the review articles evaluated. Finally, we included 42 original articles in the present study.
Probiotics vs caries: human studies over the years
Lactobacillus genus
Studies evaluating probiotics in caries were first developed in 2001. Näse et al. [14] observed that children presented lower caries incidence after consumption of milk containing Lactobacillus rhamnosus GG, compared with consumption of milk without probiotics. This research group also evaluated the effect of probiotics on caries prevention during 3 weeks. It was observed that the consumption of cheese supplemented with probiotics Lactobacillus rhamnosus GG ATCC 53103 and Lactobacillus rhamnosus LC 705 by young adults reduced by 20 and 27% the oral concentration of Streptococcus mutans and yeasts, respectively. This effect was observed only after, but not during the treatment, evidencing that the therapeutic effect occurs after probiotic colonization in the oral cavity [10].
Similar results were obtained by Glavina et al. [21] after administering yogurt containing Lactobacillus rhamnosus GG ATCC 53103 to children during 14 days. After 30 days of the intervention, there was a reduction in Streptococcus mutans count in saliva of the probiotic group, but no statistically significant difference in Lactobacillus count. Moreover, the study did not present the probiotic dose, which makes difficult data comparison.
Juneja and Kakade [22] supplemented Lactobacillus rhamnosus hct 70 for 3 weeks to children with medium and high caries activity, using milk as a vehicle. Saliva samples were collected three times: in the baseline period, immediately after intervention, and 3 weeks after the intervention. Probiotic supplementation reduced Streptococcus mutans count immediately after intervention and also after 3 weeks, where the effect was significantly more pronounced compared with baseline and placebo. Taking into account that children used non-fluoridated toothpaste and did not receive fluoride application or other dental treatments during the experimental period, the therapeutic effect observed was only due to probiotic supplementation. The consumption of milk also reduced Streptococcus mutans, indicating anticariogenic effect of this food [22].
Stecksén-Blicks et al. [23] administered Lactobacillus rhamnosus LB21 during 21 months, in combination with fluoride. A reduced incidence of caries was observed in the treated group; however, because there was no group supplemented with only probiotics (no fluoride), it is not possible to guarantee that the therapeutic effect is actually from Lactobacillus rhamnosus LB21. Differently, an elegant study in 2011 [24] evaluated the effect of milk containing probiotic and/or fluoride. Individuals were distributed into four groups: 1—receiving standard milk (placebo); 2—receiving milk containing Lactobacillus rhamnosus LB21 (107 CFU/mL) and fluoride; 3—receiving milk containing only Lactobacillus rhamnosus LB21 (107 CFU/mL); and 4—receiving milk containing only fluoride. All groups received 200 mL of milk per day for 15 months. In groups 2, 3, and 4, there were more cases of Root Caries Index (RCI) reversal, compared with placebo (group 1). There was also improvement of caries lesions in these groups. Concerning microbial counts, there was no difference between groups. Interestingly, therapeutic effects were more pronounced in groups receiving fluoride, especially group 2, that received both interventions, evidencing that probiotics can be used as adjuvants, but not replace fluoride treatment.
Recently, the probiotic Lactobacillus rhamnosus was tested again. It was observed that in the treated group, the prevalence (54.4% in the probiotic group and 65.8% in the placebo group) and the incidence of caries (9.7% new cases in the probiotic group and 24.3% in the placebo group) were lower [12].
Contrary to the abovementioned studies, Montalto et al. [25] observed that oral supplementation with a pool of Lactobacillus, containing the strains Lactobacillus sporogens, Lactobacillus bifidum, Lactobacillus bulgaricus, Lactobacillus termophilus, Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus rhamnosus, in liquid form or capsule, increased the Lactobacillus oral count, but did not alter the Streptococcus mutans count. Based on these studies, it could be suggested that the probiotic with the main effect is Lactobacillus rhamnosus, and this effect was less pronounced in the study of Montalto et al. [25] as a result of the lower administered dose compared to the other studies. In addition to this probiotic, evidence suggests that Lactobacillus casei Shirota also played an important role in reducing Streptococcus mutans oral count in children [26].
Others studies involving more than one Lactobacillus did not find beneficial effects, reinforcing the theory that supplementation with only one probiotic may be better than mixing several microorganisms, even though belonging to the same genus [27,28,29,30], although some trials do not corroborate these results [31, 32].
Besides Lactobacillus rhamnosus, other species of Lactobacillus genus were tested. Chuang et al. [22] observed that Lactobacillus paracasei GMNL-33 reduced Streptococcus mutans count when administered in adults, during 2 weeks, showing a reduction in the risk of caries. Similar results were obtained when Lactobacillus paracasei SD1 was offered to children [33] and adults [34]. In addition to the reduction of Streptococcus mutans oral count, there was an increase in Lactobacillus oral count [34] and in the salivary concentrations of neutrophil peptide 1–3, an immunological marker that is reduced in caries-susceptible children [22, 33]. Supplementation with inactivated cells of Lactobacillus paracasei DSMZ16671 also reduced Streptococcus mutans oral count [35]; however, it is worth mentioning that inactivated cells are not considered as probiotics by the World Health Organization [1, 2].
An interesting study developed by Hasslöf et al. [36] showed that supplementation with Lactobacillus paracasei F19 (LF19) did not influence caries incidence when administered in babies (aged 4 to 13 months old) and evaluated at the age of 9 years old. It was also observed that the administered probiotic was not able to colonize the oral microbiota.
A similar trial was performed by Stensson et al. [37] that aimed to evaluate whether the supplementation with Lactobacillus reuteri strain ATCC 55730 for pregnant women during the last month of gestation and in the first year of life of the neonates (108 CFU/day) improved the oral health of these children at the age of nine. It was observed that 82% of the children from the probiotic group did not present caries. This value was much higher when compared to the placebo group (only 58% without caries). The prevalence of caries lesions and gingivitis were also lower in the probiotic group. In conclusion, the authors pointed out that probiotic supplementation in pregnancy and in the first year of life reduces caries incidence at the age of 9 years old. This same probiotic also reduced Streptococcus mutans oral count, after 3 weeks of supplementation, in young adults without topical fluoride treatment within 4 weeks prior to the study [38].
Cildir et al. [39] evaluated the effect of Lactobacillus reuteri strains DSM 17938 and ATCC PTA 5289 (1 × 108 CFU/day) in children (4–12 years) with cleft lip/palate during 25 days, but there was no statistically significant difference in the salivary count of Streptococcus mutans and Lactobacillus with the treatment. Equally, these strains did not profoundly affect caries lesions in adolescents, indicating that this probiotic may not have an important role in this disease [40].
Otherwise, Nishihara et al. [41] observed that supplementation with isolated Lactobacillus salivarius strains WB21 (6.7 × 108 CFU/day) and TI 2711 (2.8 × 108 CFU/day), for 2 weeks, decreased Streptococcus mutans count and increased Lactobacillus count in saliva of adults, improving oral health and reducing the caries risk. Recently, Sañudo et al. [42] observed that supplementation with inactivated cells of Lactobacillus salivarius CECT 5713 also reduced the oral count of Streptococcus mutans, but as previously mentioned, inactivated cells are not considered probiotics.
Lactobacillus brevis CD2 also reduced Streptococcus mutans oral count, plate acidogenicity, and bleeding when administered to high caries risk children for 6 weeks. Interestingly, children were instructed not to use products containing fluoride and, therefore, we could infer that the anticariogenic effect was only due to probiotic supplementation [43].
Bifidobacterium genus
As mentioned above, several studies suggested the effect of lactobacilli-derived probiotics in affecting oral ecology and reducing caries risk factors and incidence. Nevertheless, the effect of bifidobacteria supplementation was only reported in few studies. In 2005, Caglar et al. [44] observed a reduction of Streptococcus mutans oral count after short consumption (2 weeks) of yogurt containing Bifidobacterium DN-173 010 in young adults that did not use fluoride within 4 weeks prior to the study. Similar results were obtained with Bifidobacterium animalis subsp. lactis BB-12 supplementation to adults, during 10 days, using ice cream as the food matrix [30]. However, further studies did not confirm the anticariogenic effect of bifidobacteria.
Taipale et al. [45] administered Bifidobacterium animalis subsp. lactis BB-12 for babies (1–2 months), during 14.9 ± 6.7 months, and observed that probiotic supplementation did not affect the oral count of Streptococcus mutans, Lactobacillus, and yeasts at the ages of 8 months and 2 years [46]. Moreover, there was no difference in caries incidence of these children at the age of 4 years, indicating that Bifidobacterium animalis subsp. lactis BB-12 administration in the early childhood does not reduce the risk of caries [45]. Other trials also failed in demonstrating therapeutic effects of this probiotic and other species of Bifidobacterium genus on dental caries in patients with no use of fluoride [47, 48].
Interestingly, Nozari et al. [48] observed that traditional yogurt ingestion, but not yogurt containing Bifidobacterium lactis, reduced Streptococcus mutans and Lactobacillus oral count in children. Dairy products are considered food that prevents caries because they are sources of minerals, especially calcium [1, 2]. In this context, the food matrix may also have a therapeutic effect and, in some studies, it may be questionable whether the therapeutic effect is derived from the matrix or the probiotic.
Streptococcus genus
In 2009, a pilot study with 20 individuals tested the efficacy of a mouthwash containing Streptococcus oralis strain KJ3sm, Streptococcus uberis strain KJ2sm, and Streptococcus rattus strain JH145, in two doses, 106 or 108 CFU of each probiotic, during 4 weeks. The group treated with the higher dose showed a decrease in salivary Streptococcus mutans count, by 60%, compared with the baseline level. Moreover, there was a reduction of the pathogens Campylobacter rectus and Porphyromonas gingivalis in the subgingival plaque in the same group, although there was no statistically significant difference [49].
Burton et al. [22, 50] observed that Streptococcus salivarius strain M18 administration during 3 months increased its oral count, as well as reduced the Streptococcus mutans count in children with caries. Moreover, there was a reduction of bacterial plaque scores in the treated individuals, evidencing an improvement in oral health. None of the supplemented individuals had adverse effects, indicating the safety of this treatment. In other studies, the association of microorganisms from Streptococcus genus (Streptococcus uberis KJ2, Streptococcus oralis KJ3, and Streptococcus rattus JH145), in the probiotic group ProBiora3, reduced the risk of the development of caries [18, 51].
Combination or comparison between different genres
Singh et al. [52] supplemented children with ice cream containing probiotics (Bifidobacterium lactis Bb-12 ATCC27536 and Lactobacillus acidophilus La-5) or placebo during 10 days. The probiotic group presented lower Streptococcus mutans count in saliva compared with baseline, but there was no difference in Lactobacillus levels. In placebo group (ice cream without probiotic), there was also a reduction in Streptococcus mutans oral count; however, there was no statistically significant difference. This data highlighted that the dairy foods may also have a therapeutic effect. There was no statistically significant difference between probiotic and placebo groups. A similar trial also observed that these probiotics reduced Streptococcus mutans oral count in children; however, after 6 months of the intervention, Streptococcus mutans oral concentrations returned to the baseline values, evidencing that probiotic supplementation should be continuous to maintain the therapeutic effects [53]. Another studies supplementing species from Bifidobacterium and Lactobacillus genres also observed a reduction in Streptococcus mutans oral count [54, 55], even in individuals with no use of fluoride treatments [55].
Yousuf et al. [56] investigated whether there was a synergism between Bifidobacterium and Lactobacillus genres. They supplemented schoolchildren with Lactobacillus acidophilus, Bifidobacterium longum, Bifidobacterium bifidum, and Bifidobacterium lactis (group A); only with Lactobacillus acidophilus (group B); or with placebo (group C) during 3 weeks and observed a reduction of Streptococcus mutans oral count in groups A and B. Possibly, Lactobacillus acidophilus presents the main anticariogenic effect and there was no synergism between these probiotics.
In 2013, Cannon et al. [57] supplemented medium- to high-caries-risk children with Lactobacilli reuteri for 28 days (group A) or with Streptococcus uberis KJ2, Streptococcus oralis KJ3, and Streptococcus rattus JH145 during 30 days (group B). After interventions, both groups presented lower levels of Streptococcus mutans in saliva, indicating a reduction of caries risk. There was also reduction of Lactobacillus in saliva after interventions, but the authors did not discuss this result.
Table 1 summarizes the results from the abovementioned studies.
Main results
The therapeutic effects of Lactobacillus genus are better elucidated in the literature when compared with other genres (Bifidobacterium and Streptococcus). Among the main effects presented by this genus, we highlight:
-
1.
Reduction of Streptococcus mutans oral count;
-
2.
Increase in Lactobacillus oral count;
-
3.
Reduction in caries incidence.
Concerning the Bifidobacterium supplementation, the results are conflicting but most of the trials did not observe anticariogenic role of these probiotics. Only a few studies evaluated the effects of Streptococcus genus; although they presented positive effects, the data in the literature are scarce to recommend the use of these microorganisms. Some trials evaluated the effect of more than one probiotic; however, evidence suggested no synergism between microorganisms, especially regarding Lactobacillus genus.
Conclusions
Proper tooth hygiene, fluoride, and reduced consumption of sugary foods are considered effective methods to prevent and treat caries. Of these, fluoride would be the only treatment that could be replaced by probiotic supplementation. However, in several of the studies presented, there is no exclusion of this treatment. In addition, in most studies, it is not mentioned whether fluoride treatment was being applied. Only a few studies evaluated probiotics without fluoride. While some of them presented therapeutic effect of isolated probiotics, one trial showed better effects when both interventions were applied together, indicating that probiotics can be used as adjuvants in caries treatment, but are not able to replace the conventional therapy. In any case, the number of studies is scarce to recommend supplementation with probiotics as the main treatment for dental caries.
Abbreviations
- CFU:
-
Colony-forming unit
- Mesh:
-
Medical Subject Headings
- RCI:
-
Root Caries Index
References
Food and Agriculture Organization of The United States/World Health Organization (FAO/WHO). Guidelines for the evaluation of probiotics in food. 2002.
Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, et al. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–9.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14.
Raizel R, Santini E, Kopper AM, Dos Reis Filho AD. Effects of probiotics, prebiotics and synbiotics consumption on the human organism. Revista Ciência & Saúde [Internet]. 2011;4:66–74.
Coqueiro AY, Bonvini A, Tirapegui J, Rogero MM. Probiotics supplementation as an alternative method for celiac disease treatment. International Journal of Probiotics & Prebiotics [Internet]. 2017;12:23–32.
Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11(5):853-858.
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(11):1202–9. 9.e1
Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10(11):PI126–31.
Rogero MM, Bonvini A, Coqueiro AY. Recomendações de probióticos. In: Philippi ST, RDCD A, editors. Recomendações nutricionais nos estágios de vida e nas doenças crônicas não transmissíveis: Manole; 2017.
Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlström A, Meurman JH, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002;47(11):799–804.
Chuang LC, Huang CS, Ou-Yang LW, Lin SY. Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Investig. 2011;15(4):471–6.
Rodríguez G, Ruiz B, Faleiros S, Vistoso A, Marró ML, Sánchez J, et al. Probiotic compared with standard milk for high-caries children: a cluster randomized trial. J Dent Res. 2016;95(4):402–7.
Comelli EM, Guggenheim B, Stingele F, Neeser JR. Selection of dairy bacterial strains as probiotics for oral health. Eur J Oral Sci. 2002;110(3):218–24.
Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35(6):412–20.
Nikawa H, Makihira S, Fukushima H, Nishimura H, Ozaki Y, Ishida K, et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004;95(2):219–23.
Martinez RC, Bedani R, Saad SM. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: an update for current perspectives and future challenges. Br J Nutr. 2015;114(12):1993–2015.
Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G. The use of probiotic strains in caries prevention: a systematic review. Nutrients. 2013;5(7):2530–50.
Hedayati-Hajikand T, Lundberg U, Eldh C, Twetman S. Effect of probiotic chewing tablets on early childhood caries—a randomized controlled trial. BMC Oral Health. 2015;15(1):112.
Jørgensen MR, Castiblanco G, Twetman S, Keller MK. Prevention of caries with probiotic bacteria during early childhood. Promising but inconsistent findings. Am J Dent. 2016;29(3):127–31.
Gruner D, Paris S, Schwendicke F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J Dent. 2016;48:16–25.
Glavina D, Gorseta K, Skrinjarić I, Vranić DN, Mehulić K, Kozul K. Effect of LGG yoghurt on Streptococcus mutans and Lactobacillus spp. salivary counts in children. Coll Antropol. 2012;36(1):129–32.
Juneja A, Kakade A. Evaluating the effect of probiotic containing milk on salivary mutans streptococci levels. J Clin Pediatr Dent. 2012;37(1):9–14.
Stecksén-Blicks C, Sjöström I, Twetman S. Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study. Caries Res. 2009;43(5):374–81.
Petersson LG, Magnusson K, Hakestam U, Baigi A, Twetman S. Reversal of primary root caries lesions after daily intake of milk supplemented with fluoride and probiotic lactobacilli in older adults. Acta Odontol Scand. 2011;69(6):321–7.
Montalto M, Vastola M, Marigo L, Covino M, Graziosetto R, Curigliano V, et al. Probiotic treatment increases salivary counts of lactobacilli: a double-blind, randomized, controlled study. Digestion. 2004;69(1):53–6.
Yadav M, Poornima P, Roshan NM, Prachi N, Veena M, Neena IE. Evaluation of probiotic milk on salivary mutans streptococci count: an in vivo microbiological study. J Clin Pediatr Dent. 2014;39(1):23–6.
Marttinen A, Haukioja A, Karjalainen S, Nylund L, Satokari R, Öhman C, et al. Short-term consumption of probiotic lactobacilli has no effect on acid production of supragingival plaque. Clin Oral Investig. 2012;16(3):797–803.
Keller MK, Twetman S. Acid production in dental plaque after exposure to probiotic bacteria. BMC Oral Health. 2012;12:44.
Keller MK, Hasslöf P, Dahlén G, Stecksén-Blicks C, Twetman S. Probiotic supplements (Lactobacillus reuteri DSM 17938 and ATCC PTA 5289) do not affect regrowth of mutans streptococci after full-mouth disinfection with chlorhexidine: a randomized controlled multicenter trial. Caries Res. 2012;46(2):140–6.
Caglar E, Kuscu OO, Selvi Kuvvetli S, Kavaloglu Cildir S, Sandalli N, Twetman S. Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol Scand. 2008;66(3):154–8.
Caglar E, Kavaloglu SC, Kuscu OO, Sandalli N, Holgerson PL, Twetman S. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Investig. 2007;11(4):425–9.
Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008;18(1):35–9.
Wattanarat O, Makeudom A, Sastraruji T, Piwat S, Tianviwat S, Teanpaisan R, et al. Enhancement of salivary human neutrophil peptide 1-3 levels by probiotic supplementation. BMC Oral Health. 2015;15:19.
Teanpaisan R, Piwat S. Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: a randomized placebo-controlled trial. Clin Oral Investig. 2014;18(3):857–62.
Holz C, Alexander C, Balcke C, Moré M, Auinger A, Bauer M, et al. Lactobacillus paracasei DSMZ16671 reduces mutans streptococci: a short-term pilot study. Probiotics Antimicrob Proteins. 2013;5:259–63.
Hasslöf P, West CE, Videhult FK, Brandelius C, Stecksén-Blicks C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 2013;47(6):559–65.
Stensson M, Koch G, Coric S, Abrahamsson TR, Jenmalm MC, Birkhed D, et al. Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. 2014;48(2):111–7.
Caglar E, Cildir SK, Ergeneli S, Sandalli N, Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006;64(5):314–8.
Cildir SK, Sandalli N, Nazli S, Alp F, Caglar E. A novel delivery system of probiotic drop and its effect on dental caries risk factors in cleft lip/palate children. Cleft Palate Craniofac J. 2012;49(3):369–72.
Keller MK, Nøhr Larsen I, Karlsson I, Twetman S. Effect of tablets containing probiotic bacteria (Lactobacillus reuteri) on early caries lesions in adolescents: a pilot study. Benef Microbes. 2014;5(4):403–7.
Nishihara T, Suzuki N, Yoneda M, Hirofuji T. Effects of Lactobacillus salivarius-containing tablets on caries risk factors: a randomized open-label clinical trial. BMC Oral Health. 2014;14:110.
Sañudo AI, Luque R, Díaz-Ropero MP, Fonollá J, Bañuelos Ó. In vitro and in vivo anti-microbial activity evaluation of inactivated cells of Lactobacillus salivarius CECT 5713 against Streptococcus mutans. Arch Oral Biol. 2017;84:58–63.
Campus G, Cocco F, Carta G, Cagetti MG, Simark-Mattson C, Strohmenger L, et al. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin Oral Investig. 2014;18(2):555–61.
Caglar E, Sandalli N, Twetman S, Kavaloglu S, Ergeneli S, Selvi S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand. 2005;63(6):317–20.
Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Administration of Bifidobacterium animalis subsp. lactis BB-12 in early childhood: a post-trial effect on caries occurrence at four years of age. Caries Res. 2013;47(5):364–72.
Taipale T, Pienihäkkinen K, Salminen S, Jokela J, Söderling E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 2012;46(1):69–77.
Pinto GS, Cenci MS, Azevedo MS, Epifanio M, Jones MH. Effect of yogurt containing Bifidobacterium animalis subsp. lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 2014;48(1):63–8.
Nozari A, Motamedifar M, Seifi N, Hatamizargaran Z, Ranjbar MA. The effect of Iranian customary used probiotic yogurt on the children’s salivary cariogenic microflora. J Dent (Shiraz). 2015;16(2):81–6.
Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH, Hillman JD. Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J Appl Microbiol. 2009;107(2):682–90.
Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol. 2013;62(Pt 6):875–84.
Cortés-Dorantes N, Ruiz-Rodríguez MS, Karakowsky-Kleiman L, Garrocho-Rangel JA, Sánchez-Vargas LO, Pozos-Guillén AJ. Probiotics and their effect on oral bacteria count in children: a pilot study. Eur J Paediatr Dent. 2015;16(1):56–60.
Singh RP, Damle SG, Chawla A. Salivary mutans streptococci and lactobacilli modulations in young children on consumption of probiotic ice-cream containing Bifidobacterium lactis Bb12 and Lactobacillus acidophilus La5. Acta Odontol Scand. 2011;69(6):389–94.
Ashwin D, Ke V, Taranath M, Ramagoni NK, Nara A, Sarpangala M. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6-12 years of age: a randomized controlled double blind study with six-months follow up. J Clin Diagn Res. 2015;9(2):ZC06–9.
Mahantesha T, Reddy KM, Kumar NH, Nara A, Ashwin D, Buddiga V. Comparative study of probiotic ice cream and probiotic drink on salivary Streptococcus mutans levels in 6-12 years age group children. J Int Oral Health. 2015;7(9):47–50.
Ghasemi E, Mazaheri R, Tahmourespour A. Effect of probiotic yogurt and xylitol-containing chewing gums on salivary S mutans count. J Clin Pediatr Dent. 2017;41(4):257–63.
Yousuf A, Nagaraj A, Ganta S, Sidiq M, Pareek S, Vishnani P, et al. Comparative evaluation of commercially available freeze dried powdered probiotics on mutans streptococci count: a randomized, double blind, clinical study. J Dent (Tehran). 2015;12(10):729–38.
Cannon M, Trent B, Vorachek A, Kramer S, Esterly R. Effectiveness of CRT at measuring the salivary level of bacteria in caries prone children with probiotic therapy. J Clin Pediatr Dent. 2013;38(1):55–60.
Funding
The authors would like to thank The São Paulo Research Foundation (FAPESP 2016/04910-0 and 2016/11360-6), the Brazilian National Council for Scientific and Technological Development (CNPq) (154403/2016-4), and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
The search of the studies and the initial manuscript preparation were performed by AYC, AB, and RR and revised by JT. The final manuscript version was revised by MMR. All authors agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Coqueiro, A.Y., Bonvini, A., Raizel, R. et al. Probiotic supplementation in dental caries: is it possible to replace conventional treatment?. Nutrire 43, 6 (2018). https://doi.org/10.1186/s41110-018-0064-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-018-0064-3