Abstract
Metabolism is a fundamental biological process composed of a series of reactions catalyzed by metabolic enzymes. Emerging evidence demonstrates that the aberrant signaling in cancer cells induces nonmetabolic functions of metabolic enzymes in many instrumental cellular activities, which involve metabolic enzyme-mediated protein post-translational modifications, such as phosphorylation, acetylation, and succinylation. In the most well-researched literatures, metabolic enzymes phosphorylate proteins rather than their metabolites as substrates. Some metabolic enzymes have altered subcellular localization, which allows their metabolic products to directly participate in nonmetabolic activities. This review discusses how these findings have deepened our understanding on enzymes originally classified as metabolic enzymes, by highlighting the nonmetabolic functions of several metabolic enzymes responsible for the development of cancer, and evaluates the potential for targeting these functions in cancer treatment.
Similar content being viewed by others
Introduction
Metabolism, regulated by metabolic enzymes, supports cell growth and proliferation by providing energy for cellular activities and synthesizing the molecular building blocks for production of amino acids, nucleotides, and lipids. Metabolic enzymes are responsible for specific chemical reactions on metabolites, which takes place under strict regulation at specific subcellular locations in the metabolic cascades. Metabolic enzymes, such as carboxylases, dehydrogenases, lipoxygenases, oxidoreductases, kinases, lyses, and transferases carry out a wide range of catalytic activities and are responsible for a variety of cellular functions necessary for cellular homeostasis and survival.
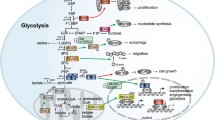
Recent literatures have determined that some metabolic enzymes possess nonmetabolic activities that are critical in the development of cancer. These nonmetabolic activities can be divided into two categories. First, metabolic enzymes that use non-metabolites as substrates to catalyze reactions that are distinct from the metabolic reactions in which they were originally characterized to work on. For instance, several metabolic enzymes use proteins as substrates and function as protein kinases to phosphorylate these protein substrates, thereby regulating diverse functions [1]. Second, metabolic enzymes that translocate from their original subcellular compartments to different organelles, where their metabolite products are directly used for protein modifications or acting as instrumental regulators for other proteins. For instance, mitochondrial α-ketoglutarate dehydrogenase (α-KGDH) that translocates to the nucleus and produces succinyl-coenzyme A (CoA), which is used by the histone acetyltransferase, lysine acetyltransferase 2A (KAT2A), to succinylate histone H3 [2, 3]. In addition, mitochondrial fumarase, when translocated to the nucleus, produces fumarate that inhibits lysine demethylase 2B (KDM2B) histone demethylase activity and enhances the methylation of histone H3 and the repair of damaged DNA [4]. This review summarizes the recent findings regarding these nonmetabolic functions of metabolic enzymes and highlights the implication of these functions in cancer development.
Metabolic enzymes function as protein kinases
Protein kinases are critical regulators of intracellular signal transduction pathways that mediate various cellular processes in both unicellular and multicellular organisms. They can directly transfer the γ-phosphate from adenosine triphosphate (ATP) to specific tyrosine (Tyr), serine (Ser), threonine (Thr), and histidine (His) residues on substrate proteins, thereby altering the functions of these substrates. More than 500 protein kinases have been identified in humans, constituting of about 1.7% of all human genes [5]. Recent studies have demonstrated that several metabolic enzymes, such as pyruvate kinase M2 (PKM2), phosphoglycerate kinase 1 (PGK1), ketohexokinase-A (KHK-A), hexokinases (HK), nucleoside diphosphate kinase (NDPK or NDK), and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4), have unexpected protein kinase activities and play significant roles in nonmetabolic cellular functions. These new studies expand the family of protein kinases and provide new insights into the integrated regulation of cell metabolism and other cellular processes.
PKM2
Pyruvate kinase (PK) catalyzes the final rate-limiting step of glycolysis and converts phosphoenolpyruvate (PEP) to pyruvate by transferring a phosphate group from PEP to adenosine diphosphate (ADP), producing ATP. It has four isoforms: PKL, PKR, PKM1, and PKM2. PKM2 is highly expressed in cancer cells [6]. Besides, although PKM1 has a higher glycolytic activity than PKM2, only the protein kinase activity of PKM2 has been described till present. PKM2 is involved in the regulation of gene expression, mitosis, apoptosis, and other critical cellular activities that promote aerobic glycolysis and tumor growth [7,8,9].
PKM2’s protein kinase activity was initially identified from the phosphorylation of histone H3 at Thr11 and that of signal transducer and activator of transcription 3 (STAT3) at Tyr705. In the nucleus, PKM2-mediated histone H3 phosphorylation promotes β-catenin- and c-Myc-mediated gene expression, which enhances aerobic glycolysis and promotes the proliferation of tumor cells [10,11,12,13,14]. During mitosis, PKM2 binds to the spindle checkpoint protein Bub3 and phosphorylate it at Tyr207 to enable the interaction of the Bub3–Bub1 complex with kinetochores, which is essential for the mitotic/spindle-assembly checkpoint, accurate chromosome segregation, and tumorigenesis [15]. PKM2 also phosphorylates myosin light chain 2 (MLC2) at Tyr118, primes the binding of Rho-associated protein kinase 2 (ROCK2) to MLC2 and the phosphorylation of ROCK2–MLC2 complex at Ser15, to allow the interaction between myosin II with actin, which is required for the contractile function of the actomyosin complex at the cleavage furrow, completion of the cytokinesis process, and proliferation of tumor cells [16]. In addition, it has been found that in hepatocellular carcinoma (HCC), PKM2 phosphorylates the sterol regulatory element-binding proteins (SREBPs) at Thr59, activates lipid biosynthesis, and promotes the proliferation of the cancer cells [17]. Apart from its functions in regulating gene expression and cell cycle progression, PKM2 has been demonstrated to phosphorylate serine/threonine kinase 1 (AKT1) at Ser202/203 to release it from the regulatory-associated protein of mTOR (raptor), which subsequently promotes hormonal and nutrient signaling-independent activation of the mammalian target of rapamycin complex 1 (mTORC1), to provide survival and proliferation advantages over normal cell upon stimulus by the epidermal growth factor receptor (EGFR)-dependent activation of nuclear factor kappa enhancer binding protein (NF-κB) [6, 18].
In response to oxidative stress, PKM2 promotes cell survival by translocating into the mitochondria, phosphorylating the apoptosis regulator Bcl2 at Thr69 to stabilize Bcl2, which blocks its association with Cul3-based E3 ligase to prevent the degradation of Bcl2 and promotes the resistance of tumor cells against apoptosis [19]. Further, as PKM2 can also phosphorylate the synaptosome-associated protein 23 (SNAP23) at Ser95, resulting in the formation of the soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE) complex, this demonstrates that PKM2 has contribution in the remodeling of the tumor microenvironments by promoting tumor cell exosome secretions, which are necessary for the docking of tumor cells to plasma membranes [20]. A recent report has shown that in pancreatic ductal adenocarcinoma, PKM2 could phosphorylate the serine/threonine protein kinase PAK2 at Ser20, Ser141, and Ser 192/197 to recruit heat shock protein 90 for increasing the stability of the PAK2 protein, thereby promoting invasion and metastasis of the pancreatic cancerous cells [21]. Further, PKM2 was observed to promote genomic instability, an important hallmark of cancer development and progression due to increased interruption of DNA repair, in breast cancer cells breast cancer since it was demonstrated that nuclear PKM2 could interact with and directly phosphorylate histone H2AX at Ser139 under DNA damaged conditions to induce chromosomal aberrations and promote cancer cell proliferation [22]. Furthermore, phosphoproteomic studies in yeast and mammalian cells have revealed that hundreds of additional proteins, including many protein kinases such as extracellular signal-regulated kinase (ERK), AKT1 substrate 1 (AKT1S1), BLK, can be phosphorylated by PKM2 [18, 23, 24], suggesting that PKM2’s protein kinase activities have instrumental roles in a wide array of cellular functions.
PGK1
PGK1 is an enzyme responsible for the first ATP-generating step in the glycolysis pathway and is highly expressed in many types of cancer [25]. It catalyzes the reversible conversion of 1,3-diphosphoglycerate and ADP to 3-phosphoglycerate and ATP, respectively [25]. And similar to PKM2, PGK1 also has protein kinase activity to phosphorylate protein substrates. We found that mitochondria-translocated PGK1 uses ATP as a phosphate donor to directly phosphorylate and activate pyruvate dehydrogenase kinase isozyme 1 (PDHK1) at Thr338. The subsequent PDHK1-mediated phosphorylation of pyruvate dehydrogenase E1α at Ser293 inhibits the pyruvate dehydrogenase complex (PDC) and the conversion of pyruvate and CoA to acetyl-CoA in the mitochondria, which suppresses the mitochondrial pyruvate oxidation and increases lactate production from pyruvate in the cytosol. Thus, mitochondrial pyruvate metabolism suppressed by PGK1 couples with the upregulation of glycolytic gene expression mediated by nuclear PKM2 to promote the Warburg effect and tumorigenesis [26]. In addition, the protein kinase activity of PGK1 is involved in the initiation of autophagy, important for homogenizing cell homeostasis. When tumors start outgrowing existing vasculature, this result in glutamine deprivation and hypoxia within the tumor cells and ARD1 acetyl-transferase acetylates PGK1 at Lys388, which in turn phosphorylates Beclin1 at Ser30, leading to a conformational change and activation of class III phosphatidylinositol (PI) 3-kinase (VPS34) to produce phosphatidylinositol 3-phosphate (PI(3)P), facilitating the initiation of tumor-autophagy and promotes tumor development [27]. Thus, targeting PGK1 can be an attractive therapeutic approach for cancer treatment.
KHK-A
KHK, also known as fructokinase, is responsible for the first rate-limiting enzymatic reaction in fructose metabolism. KHK catalyzes the transfer of a phosphate group from ATP to fructose, producing fructose 1-phosphate (F1P) and ADP. Aldolase then catalyzes the F1P into dihydroxyacetone phosphate and glyceraldehyde, which are subsequently converged into the glycolysis pathway [28]. Among the alternatively spliced isoforms of the precursor ribonucleic acid (RNA) of KHK, highly active KHK-C, but not inactive KHK-A, is highly expressed in the liver, kidneys, and pancreas [29]. In HCC cells, c-Myc induces the expression of heterogeneous nuclear ribonuclear proteins H1 (HnRNPH1) and nRNPH2, which regulates the splicing of the KHK precursor mRNA and switches KHK-C to KHK-A. The expression of KHK-A, which has a much lower activity toward phosphorylation of fructose, slows the fructose catabolism rates, ATP consumption, and reactive oxygen species production in HCC cells [30]. Importantly, instead of binding to fructose, KHK-A interacts with the rate-limiting enzyme phosphoribosyl pyrophosphate synthetase 1 (PRPS1) in the de novo nucleic acid synthesis pathway and acts as a protein kinase to directly phosphorylate PRPS1 at Thr225. This phosphorylation blocks the ADP binding-mediated inhibition of PRPS1, resulting in elevated de novo nucleic acid synthesis via the constitutive activation of PRPS1 and enhancing HCC cell proliferation and liver tumor growth in mice. KHK-A expression and PRPS1 Thr225 phosphorylation levels are positively correlated with each other in human HCC specimens and are inversely correlated with survival in HCC patients, indicating that KHK-A-dependent PRPS1 phosphorylation is pivotal in HCC progression [28, 30].
HK
Most cancers ensure sufficient energy supplies via glycolysis which are the basis for their growth and proliferation. However, the systemic inhibition of glycolysis as an anti-cancer approach would result in considerable adverse effects since normal cells also supplements themselves with energy through glycolysis. Therefore, the selective inhibition of cancer-driven glycolysis has been investigated for clinical cancer therapy and HK was proposed as a therapeutic target. HK enzymes have been found to be essential in the first step of the glucose metabolism pathway as they catalyse the phosphorylation of glucose to produce glucose 6-phosphate by using ATP as the phosphate donor. Phosphoamino acid analyses revealed that HK1 purified from rat brains can be autophosphorylated at serine, threonine, and tyrosine residues [31], and in vitro phosphorylation assays showed that HK1 can phosphorylate itself and purified histone H2A [32]. However, the different isoforms of HK need to be characterized and whether HK1 or other HK isoforms act as protein kinases in vivo remains unclear, as does the physiological role of any such phosphorylation activity in the regulation of cellular activities associated with cancer development.
NDPK1/2
NDPK is a ubiquitous enzyme in mammals. It catalyzes the conversion of nucleoside diphosphates (NDPs) into nucleoside triphosphates (NTPs) by transferring the γ-phosphate group from the 5′-triphosphate nucleotides to the 5′-diphosphate nucleotides [33]. In this process, NDPK uses an NTP (usually ATP) and autophosphorylate itself at a highly conserved histidine residue in its active site. The phosphate group is then transferred from the phosphohistidine to an NDP molecule or to a histidine on the substrate protein [34]. Several proteins have been identified as substrates of NDPK-A and NDPK-B protein kinase activity. For instance, NDPK-A phosphorylates the histidine at the catalytic site of ATP citrate lyase (ACLY) and regulates ACLY-dependent acetyl-CoA production for fatty acid synthesis [35]. NDPK-B can form a complex with G protein βγ dimers and phosphorylate the Gβ subunit at His266. The phosphate group is then transferred to guanosine diphosphate (GDP), leading to the formation of guanosine triphosphate (GTP) and the activation of G protein [36]. NDPK-B also phosphorylates the Ca2+-activated K+ channel KCa3.1 at His358. This phosphorylation relieves the copper-dependent inhibition of KCa3.1 channel function and promotes subsequent activation of CD4+ T cells [37, 38]. In addition, NDPK-B phosphorylates the C-terminal tail of transient receptor potential vanilloid 5 at His711 and regulates urinary Ca2+ excretion by mediating active Ca2+ reabsorption in the distal convoluted tubule of the kidney [39]. However, like that of HK, the role of the protein histidine-kinase activity of NDPK in the development of cancer are yet to be uncovered.
PFKFB4
In humans, four genes encode phosphofructokinase two proteins: PFKFB1, PFKFB2, PFKFB3, and PFKFB4. These proteins vary dramatically in their tissue expression, regulation, and kinase-to-phosphatase activity. Phosphofructokinase 2 promotes glycolysis by phosphorylation to generate fructose 6-phosphate, an allosteric activator of phosphofructokinase 1. In a recent study, PFKFB4 was found to have protein kinase activity that phosphorylates steroid receptor coactivator-3 (SRC3) at the Ser857 [40], which promotes the association of SRC3 with the activating transcription factor 4 (ATF4) to induce gene expression of the enzymes transketolase, adenosine monophosphate deaminase-1 (AMPD1), and xanthine dehydrogenase (XDH), involved in the purine metabolism to promote metastasis. As such, PFKFB4-activated SRC3 activation drives glucose flux towards the pentose phosphate pathway, enhances purine synthesis, and promotes tumor progression [41].
Metabolic enzymes regulate cellular activity via their metabolite products
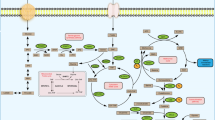
Metabolic enzymes can form complexes with other proteins and regulate the functions of these proteins via their metabolite products. Our research group and others have demonstrated that nucleus-localized acetyl-CoA synthetase short-chain family member 2 (ACSS2), ACLY, PDC, α-KGDH, and fumarase are in complex with chromatin modulators and regulate gene expression and DNA repair through their metabolite products.
ACSS2
Histone acetylation requires nuclear acetyl-CoA and is critical for gene expression. ACSS2 is a non-mitochondrial source of acetyl-CoA, which localizes in the cytosol and the nucleus, and catalyzes the ligation of acetate, both from exogenous sources and recycled histone deacetylase reactions, to CoA to produce acetyl-CoA [42, 43]. In response to stresses such as hypoxia the amount of nutrients becomes limited, conditions commonly observed in tumor microenvironments, and cytosolic ACSS2 is translocated to the nucleus, where it forms a complex with transcription factor EB (TFEB), locally producing acetyl-CoA for histone H3 acetylation in the promoter regions of TFEB-regulated autophagosomal and lysosomal genes. The expression of these genes promotes glucose deprivation-induced autophagy and lysosomal biogenesis, tumor cell survival, and proliferation [43].
ACLY
ACLY, which catalyzes the conversion of oxidation-derived mitochondrial citrate into acetyl-CoA and oxaloacetate, provides an important source of cytosolic acetyl-CoA. Nevertheless, ACLY is also found in the nucleus, and the spatial and temporal control of nuclear ACLY-dependent acetyl-CoA production plays an important role in histone acetylation and regulation of gene expression [44,45,46]. The identification of co-regulators that are in complex with ACLY will shed further light on the selective regulation of gene expression.
PDC
In addition to ACLY and ACSS2, PDC, which is primarily located in the mitochondria, is another non-mitochondrial source of acetyl-CoA. Upon treatment of cells with serum and epidermal growth factor, PDC translocates into the nucleus and generates acetyl-CoA for histone acetylation [47]. As with ACLY, the complexed proteins with PDC that control gene expression remain undetermined.
α-KGDH
Mitochondrial α-KGDH catalyzes the conversion of α-ketoglutarate into succinyl-CoA in the tricarboxylic acid cycle. In our recent studies, we have demonstrated that a small percentage of α-KGDH is located in the nucleus [2]. Nuclear α-KGDH interacts with KAT2A to form a high-order assembly and locally converts α-ketoglutarate and CoA into succinyl-CoA, which can be used by KAT2A to succinylate histone H3 [2, 3]. Nuclear α-KGDH-coupled KAT2A functions as a histone succinyltransferase to regulate gene expression, which is pivotal in promoting cancer cell proliferation and tumor growth [2, 3].
Fumarase
Fumarase catalyzes the reversible reaction of fumarate to malate in the mitochondria and cytosol. Upon induction of DNA damage by ionizing radiation, fumarase translocates from the cytosol into the nucleus. In the nucleus, DNA-dependent protein kinase (DNA-PK)-phosphorylated fumarase binds to histone H2A.Z at irradiation-induced double-strand break regions and locally produces fumarate, which inhibits α-ketoglutarate-dependent KDM2B activity. Thus, H2A.Z-complexed fumarase increases the demethylation of histone H3K36 and accumulation of the DNA-PK complex at DNA damage foci to induce nonhomologous end joining DNA repair and enhance cell survival. In addition, chromatin-associated fumarase can regulate gene transcription. Under conditions of glucose deprivation, AMP-activated protein kinase-phosphorylated fumarase binds to the transcription factor ATF2 and translocates to ATF2-regulated gene promoter regions, where fumarase-catalyzed fumarate inhibits KDM2A, increases histone H3K36 demethylation and gene expression to inhibit cell growth. Thus, the role of fumarase in DNA damage repair and regulation of gene expression depends on its post-translational modifications and interacting proteins.
Conclusions and perspective
Recent studies have demonstrated that metabolic enzymes have nonmetabolic activity. The characterization of this activity has expanded our understanding on the integrated spatial and temporal control of metabolic and nonmetabolic processes [48, 49]. The characterization of multiple types of protein kinase activities of PKM2, PGK1, KHK-A, HK, PFKFB4, and NDPK-A/B makes the identification of the protein kinase activity of other metabolic enzymes a reasonable quest. The ability of these metabolic enzymes to transfer their phosphate groups to substrate proteins instead of that of their substrate metabolites indicates the non-ridged substrate selectivity in the chemical reactions that are catalyzed by metabolic enzymes.
In addition to these alternative enzymatic activities, altered subcellular localization also contributes to the involvement of metabolic enzymes in nonmetabolic processes [48, 50]. Metabolic reactions takes place in specific subcellular compartments to ensure that the metabolites enter into the correct metabolic cascade. Most metabolic enzyme-mediated nonmetabolic reactions occur outside the locations of the originally identified metabolic reactions. Importantly, the nonmetabolic functions of metabolic enzymes are crucial to tumorigenesis and cancer progression. Advances in our understanding of the nonmetabolic activities of metabolic enzymes would lead to the development of new and specific therapeutics for improving cancer treatments.
Abbreviations
- α-KGDH:
-
α-ketoglutarate dehydrogenase
- ACLY:
-
ATP-citrate lyase
- ACSS2:
-
acetyl-CoA synthetase short chain family member 2
- ATF4:
-
cyclic AMP-dependent transcription factor
- CoA:
-
coenzyme A
- DNA-PK:
-
DNA-dependent protein kinase
- F1P:
-
fructose 1-phosphate
- HCC:
-
hepatocellular carcinoma
- HK:
-
hexokinase
- KAT2A:
-
histone acetyltransferase KAT2A
- KDM2B:
-
lysine-specific demethylase 2B
- KHK:
-
ketohexokinase
- MLC2:
-
myosin light chain 2
- NDP:
-
nucleoside diphosphate
- NDPK:
-
nucleoside diphosphate kinase
- NTP:
-
nucleoside triphosphate
- PDC:
-
pyruvate dehydrogenase complex
- PDHK1:
-
pyruvate dehydrogenase kinase isozyme 1
- PEP:
-
phosphoenolpyruvate
- PFKFB:
-
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase
- PGK1:
-
phosphoglycerate kinase 1
- PKM2:
-
pyruvate kinase M2
- PRPS1:
-
phosphoribosyl pyrophosphate synthetase 1
- SRC3:
-
steroid receptor coactivator 3
- TFEB:
-
transcription factor EB
References
Lu ZM, Hunter T. Metabolic kinases moonlighting as protein kinases. Trends Biochem Sci. 2018;43(4):301–10. https://doi.org/10.1016/j.tibs.2018.01.006.
Wang YG, Guo YR, Liu K, Yin Z, Liu R, Xia Y, et al. KAT2A coupled with the alpha-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552(7684):273. https://doi.org/10.1038/nature25003.
Wang Y, Guo YR, Xing D, Tao YJ, Lu Z. Supramolecular assembly of KAT2A with succinyl-CoA for histone succinylation. Cell Discov. 2018. https://doi.org/10.1038/s41421-018-0048-8.
Jiang Y, Qian X, Shen J, Wang Y, Li X, Liu R, et al. Local generation of fumarate promotes DNA repair through inhibition of histone H3 demethylation. Nat Cell Biol. 2015;17(9):1158–68. https://doi.org/10.1038/ncb3209.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–34. https://doi.org/10.1126/science.1075762.
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell. 2012;48(5):771–84. https://doi.org/10.1016/j.molcel.2012.09.028.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3. https://doi.org/10.1038/nature06734.
Guminska M, Stachurska MB, Ignacak J. Pyruvate-kinase isoenzymes in chromatin extracts of ehrlich ascites tumor, morris hepatoma-7777 and normal mouse and rat livers. Biochem Biophys Acta. 1988;966(2):207–13. https://doi.org/10.1016/0304-4165(88)90113-4.
Mellati AA, Yucel M, Altinors N, Gunduz U. Regulation of M2-type pyruvate kinase from human meningioma by allosteric effectors fructose 1,6 diphosphate and l-alanine. Cancer Biochem Biophys. 1992;13(1):33–41.
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–304. https://doi.org/10.1038/ncb2629.
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150(4):685–96. https://doi.org/10.1016/j.cell.2012.07.018.
Gao XL, Wang HZ, Yang JJ, Liu XW, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. https://doi.org/10.1016/j.molcel.2012.01.001.
Yang WW, Xia Y, Ji HT, Zheng YH, Liang J, Huang WH, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118-U289. https://doi.org/10.1038/nature10598.
Yang WW, Lu ZM. Nuclear PKM2 regulates the Warburg effect. Cell Cycle. 2013;12(19):3154–8. https://doi.org/10.4161/cc.26182.
Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 2014;53(1):75–87. https://doi.org/10.1016/j.molcel.2013.11.001.
Jiang Y, Wang Y, Wang T, Hawke DH, Zheng Y, Li X, et al. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat Commun. 2014;5:5566. https://doi.org/10.1038/ncomms6566.
Zhao XP, Zhao L, Yang H, Li JJ, Min XJ, Yang FJ, et al. Pyruvate kinase M2 interacts with nuclear sterol regulatory element-binding protein 1a and thereby activates lipogenesis and cell proliferation in hepatocellular carcinoma. J Biol Chem. 2018;293(17):6623–34. https://doi.org/10.1074/jbc.RA117.000100.
He CL, Bian YY, Xue Y, Liu ZX, Zhou KQ, Yao CF, et al. Pyruvate kinase M2 activates mTORC1 by phosphorylating AKT1S1. Sci Rep. 2016;6:21524. https://doi.org/10.1038/srep21524.
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017;27(3):329–51. https://doi.org/10.1038/cr.2016.159.
Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041. https://doi.org/10.1038/ncomms14041.
Cheng TY, Yang YC, Wang HP, Tien YW, Shun CT, Huang HY, et al. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene. 2018;37(13):1730–42. https://doi.org/10.1038/s41388-017-0086-y.
Xia L, Qin K, Wang XR, Wang XL, Zhou AW, Chen GQ, et al. Pyruvate kinase M2 phosphorylates H2AX and promotes genomic instability in human tumor cells. Oncotarget. 2017;8(65):109120–34. https://doi.org/10.18632/oncotarget.22621.
Keller KE, Doctor ZM, Dwyer ZW, Lee YS. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell. 2014;53(5):700–9. https://doi.org/10.1016/j.molcel.2014.02.015.
Li SS, Swanson SK, Gogol M, Florens L, Washburn MP, Workman JL, et al. Serine and SAM responsive complex SESAME regulates histone modification crosstalk by sensing cellular metabolism. Mol Cell. 2015;60(3):408–21. https://doi.org/10.1016/j.molcel.2015.09.024.
Li XJ, Zheng YH, Lu ZM. PGK1 is a new member of the protein kinome. Cell Cycle. 2016;15(14):1803–4. https://doi.org/10.1080/15384101.2016.1179037.
Li XJ, Jiang YH, Meisenhelder J, Yang WW, Hawke DH, Zheng YH, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA Cycle in tumorigenesis. Mol Cell. 2016;61(5):705–19. https://doi.org/10.1016/j.molcel.2016.02.009.
Qian X, Li XJ, Cai QS, Zhang CB, Yu QJ, Jiang YH, et al. Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol Cell. 2017;65(5):917. https://doi.org/10.1016/j.molcel.2017.01.027.
Li XJ, Qian X, Lu ZM. Fructokinase A acts as a protein kinase to promote nucleotide synthesis. Cell Cycle. 2016;15(20):2689–90. https://doi.org/10.1080/15384101.2016.1204861.
Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, MacLean PS, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA. 2012;109(11):4320–5. https://doi.org/10.1073/pnas.1119908109.
Li XJ, Qian X, Peng LX, Jiang YH, Hawke DH, Zheng YH, et al. A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat Cell Biol. 2016;18(5):561. https://doi.org/10.1038/ncb3338.
Adams V, Schieber A, Mccabe ERB. Hexokinase autophosphorylation: identification of a new dual-specificity protein-kinase. Biochem Med Metab B. 1994;53(1):80–6. https://doi.org/10.1006/bmmb.1994.1061.
Adams V, Griffin LD, Gelb BD, Mccabe ERB. Protein-kinase activity of rat-brain hexokinase. Biochem Biophys Res Commun. 1991;177(3):1101–6. https://doi.org/10.1016/0006-291x(91)90652-N.
Boissan M, Dabernat S, Peuchant E, Schlattner U, Lascu I, Lacombe ML. The mammalian Nm23/NDPK family: from metastasis control to cilia movement. Mol Cell Biochem. 2009;329(1–2):51–62. https://doi.org/10.1007/s11010-009-0120-7.
Attwood PV, Wieland T. Nucleoside diphosphate kinase as protein histidine kinase. N-S Arch Pharmacol. 2015;388(2):153–60. https://doi.org/10.1007/s00210-014-1003-3.
Wells TNC. Atp-citrate lyase from rat-liver: characterization of the citryl–enzyme complexes. Eur J Biochem. 1991;199(1):163–8. https://doi.org/10.1111/j.1432-1033.1991.tb16105.x.
Cuello F, Schulze RA, Heemeyer F, Meyer HE, Lutz S, Jakobs KH, et al. Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and G beta subunits: complex formation of NDPK B with G beta gamma dimers and phosphorylation of His-266 in G beta. J Biol Chem. 2003;278(9):7220–6. https://doi.org/10.1074/jbc.M210304200.
Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, Johnson AK, et al. Histidine phosphorylation of the potassium channel KCa31 by nucleoside diphosphate kinase B is required for activation of KCa31 and CD4 T cells. Mol Cell. 2006;24(5):665–75. https://doi.org/10.1016/j.molcel.2006.11.012.
Srivastava S, Panda S, Li Z, Fuhs SR, Hunter T, Thiele DJ, et al. Histidine phosphorylation relieves copper inhibition in the mammalian potassium channel KCa3.1. Elife. 2016;5:e16093. https://doi.org/10.7554/elife.16093.
Cai XJ, Srivastava S, Surindran S, Li Z, Skolnik EY. Regulation of the epithelial Ca2+ channel TRPV5 by reversible histidine phosphorylation mediated by NDPK-B and PHPT1. Mol Biol Cell. 2014;25(8):1244–50. https://doi.org/10.1091/mbc.E13-04-0180.
Dasgupta S, Rajapakshe K, Zhu BK, Nikolai BC, Yi P, Putluri N, et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature. 2018;556(7700):249. https://doi.org/10.1038/s41586-018-0018-1.
Ros S, Floter J, Kaymak I, Da Costa C, Houddane A, Dubuis S, et al. 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 is essential for p53-null cancer cells. Oncogene. 2017;36(23):3287–99. https://doi.org/10.1038/onc.2016.477.
Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16(11):708–17. https://doi.org/10.1038/nrc.2016.87.
Li XJ, Yu WL, Qian X, Xia Y, Zheng YH, Lee JH, et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol Cell. 2017;66(5):684. https://doi.org/10.1016/j.molcel.2017.04.026.
Sivanand S, Rhoades S, Jiang QQ, Lee JV, Benci J, Zhang JW, et al. Nuclear acetyl-CoA production by ACLY promotes homologous recombination. Mol Cell. 2017;67(2):252. https://doi.org/10.1016/j.molcel.2017.06.008.
Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP: citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP: citrate lyase by phosphorylated sugars. Biochemistry. 2000;39(5):1169–79. https://doi.org/10.1021/bi992159y.
Sivanand S, Viney I, Wellen KE. Spatiotemporal control of acetyl-CoA metabolism in chromatin regulation. Trends Biochem Sci. 2018;43(1):61–74. https://doi.org/10.1016/j.tibs.2017.11.004.
Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158(1):84–97. https://doi.org/10.1016/j.cell.2014.04.046.
Li X, Egervari G, Wang Y, Berger SL, Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol. 2018. https://doi.org/10.1038/s41580-018-0029-7.
Schvartzman JM, Thompson CB, Finley LWS. Metabolic regulation of chromatin modifications and gene expression. J Cell Biol. 2018;217(7):2247–59. https://doi.org/10.1083/jcb.201803061.
Wagner GR, Bhatt DP, O’Connell TM, Thompson JW, Dubois LG, Backos DS, et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 2017;25(4):823. https://doi.org/10.1016/j.cmet.2017.03.006.
Authors’ contributions
SL and YW wrote and revised this article. Both authors read and approved the final manuscript.
Acknowledgements
We thank Amy Ninetto at the Department of Scientific Publications at MD Anderson Cancer Center for the critical proof-reading of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the 2018 UT Proteomics Network Pilot Fund (to Y.W.), and the NIH/NCI Cancer Center Support Grant P30CA016672 and Brain Cancer SPORE 2P50 CA127001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lu, S., Wang, Y. Nonmetabolic functions of metabolic enzymes in cancer development. Cancer Commun 38, 63 (2018). https://doi.org/10.1186/s40880-018-0336-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-018-0336-6