Abstract
The presence of heavy metals in polluted water is known not only to cause severe harm to marine organisms, but also to terrestrial plants and animals including human beings. This research applied low-cost and environmentally benign adsorbent primed from waste orange peel (OP) for the removal of Cd(II) ions from aqueous solution via batch adsorption process. The surface properties of the orange peel powder were studied using scanning electron microscopy (SEM), X-ray spectroscopy (XRD) and Fourier transform infrared spectroscopy (FT-IR). Operational conditions like temperature, contact time, sorbent dosage, solution pH and initial adsorbate concentration were investigated. The utmost uptake of Cd(II) ion was obtained at a contact time of 120 min, initial metal concentration of 240 mg/L, adsorbent dosage of 0.04 g/L, temperature of 45 °C and solution pH of 5.5. Equilibrium results showed that the orange peel adsorbent has an adsorption capacity of 128.23 mg/g as obtained from the Langmuir isotherm. The adsorption kinetics data followed a pseudo-first-order kinetic model with correlation coefficient (R2) > 0.9 and low standard % error values. The adsorption process was found to be endothermic in nature with enthalpy of 0.0046 kJ mol−1 and entropy of-636.865 Jmol−1K−1. Results from the optimization study indicated that higher adsorbent dosage and lower Cd(II) ion concentration increased the percentage of Cd(II) ion removal. Thus, orange peel could be used in the removal of Cd(II) ion from aqueous solutions.

Similar content being viewed by others
Introduction
With the rapid development, industrialization and urbanization, massive volume of sewage sludge is being produced in the urban areas of major countries of the world (Riaz et al. 2018). Activities in the industry such as tanneries, mining and metal plating can result in the pollution of water system due to the discharge of their by-products, many of which contain heavy metals (Brinza et al. 2009; Baysal et al. 2009, Ofudje et al. 2017). While some metals are known to be essential minerals for various biochemical activities, the presence of large or even small amounts of such metals like copper, manganese, lead, cadmium, or zinc, can not only result in severe human health effects, but can equally affect the lives of animals and even plants (Brinza et al. 2009; Ofudje et al. 2013). It is well known that the processing of heavy metals by human body is very difficult since they are non-biodegradable and as such, they settle down in different internal organs and could lead to serious damage of body system (Gavrilescu 2004). Cadmium (Cd) has been reported to be one of the most poisonous elements that man could be exposed to either in the environment or at work. Also, it can as well accumulate in the human body once absorbed and is efficiently retained throughout life (Bernard 2008). It is principally poisonous to kidney causing bone demineralization while excessive exposures to cadmium could damage the proper function of the lung and increase the chance of lung cancer (Bernard, 2008).
A variety of conventional methods like reverse osmosis, electro-dialysis, ion exchange, adsorption and chemical precipitation have been documented for the elimination of heavy metals from contaminated waters (Ofudje et al. 2017, 2020; Gavrilescu et al. 2009; Sadrzadeh et al. 2008). However, some of these methods are expensive, not efficient and sometimes, could lead to the generation of sludge. Adsorption which is the adhesion of ions or molecules onto solid surface has been recognized as efficient method for heavy metals removal from contaminated water since it is cheap and eco-friendly (Gavrilescu 2004; Pintilie et al. 2007). The most commonly used adsorbent in the adsorption process is activated carbon, but its soaring cost of fabrication often limits its application (Ho et al. 2004). Thus, the search for various adsorbents with excellent adsorption properties has been the focus of some researchers in the last decades. Several adsorbents such as bone meal-derived apatite (Ofudje et al. 2020), fish scale (Adeogun et al. 2018), olive cake (Doyurum and Celik 2006), black gram husk (Saeed et al. 2005), kraft lignin (Mohan et al. 2006), tea-industry waste (Cay et al. 2004), bagasse-derived activated carbon (Mohan and Singh, 2002) and activated carbon from Filtrasorb (Kapoor et al. 1999) have been reported for their ability to eradicate cadmium ions in polluted waste water. The use of these waste agricultural by-products in the elimination of impurities from the environment has continued to gain great momentum in recent years due to the fact that they are readily available, simple, require low cost of production and do not generate secondary pollutants when compared with their conventional counterparts. Nigeria is one of the largest producers of citrus in Africa which is widely consumed across the length and breadth of the country. While the fruit juice which is extracted from the orange is an excellent source of vitamin C, a powerful natural antioxidant that builds the body immune system and also contains important phytochemicals such as synephrine, polyphenols, liminoids, hesperidin flavonoid, pectin, and sufficient amount of potassium, folacin, thiamine, niacin, calcium and magnesium, the orange peel which has little or no economic value is often discarded into the environment thus constituting environmental nuisance. With the rapid increase in population and an upsurge in demand for oranges, waste generation from orange peel is on the increase and this is worrisome. Therefore, the benefits of using orange peel as adsorbent in the elimination of cadmium ions from aqueous solution will not only serve as a means of pollution decontamination, but will also add values to the initially perceived agricultural waste. To this end, this current study presents the sorption of cadmium ions from aqueous solution by means of renewable and cheaper precursor of agricultural by-products from orange peel (OP). The adsorbent was characterized using scanning electron microscope (SEM), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR). The roles of initial concentration of adsorbates, contact time, pH, adsorbent dosage, temperature and regeneration study were investigated. Three isotherm models: Langmuir, Freundlich and Dubinin–Radushkevich isotherms were used to explain the equilibrium data, while pseudo-first order, pseudo-second order, Elovich and intraparticle diffusion kinetic models were employed to interpret the kinetic data.
Materials and methods
Adsorbent preparation
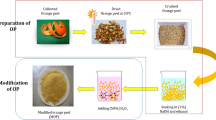
Orange peel (OP) samples were gathered from Osiele market in Abeokuta area of Ogun State, Nigeria, and were rinsed with distilled water, after which they were air dried and later oven dried at 80 °C for 12 h. Samples were further ground into fine powder, sieved with 0.5 mm sieve and stored in a container which is air tight for further analysis.
Characterization of the prepared adsorbent
Scanning electron microscope (Hitachi, Japan, S-3000H) which is made up of energy dispersive X-ray (EDX) was used to assess the surface morphology of the prepared adsorbent. Fourier transform infrared (FT-IR) spectra were performed on the OP sample in order to investigate the various functional groups present and it was done with TENSOR 27 spectrometer (Bruker, Germany) from 400 to 4000 cm−1. The crystallography of orange peel powder was investigated by Bruker D8 Advance X-ray Diffractometer with Cu Kɑ (λ = 1.5405 Å) radiation in the 2θ range from 10 to 90°.
Preparation of adsorbate solutions
Solution of 1000 mg/L of Cd(II) ion was made by weighing 0.4400 g of CdCl2.2H2O and dissolved in distilled water using a 250-mL standard flask and made to mark. Various concentrations of cadmium ions (50–300 mg/L) working standards were thereafter made from the stock solution in 250-mL standard flasks and made to mark.
Determination of effect of agitation time
The experiment was achieved at a pH of 5.5 with varying contact time of 5, 10, 15, 20, 30, 60, 120 and 240 min at a temperature of 45 °C. 0.2 g of the adsorbent was introduced into 25 mL each of the metal solution whose concentrations ranged from 50 mg/L to 300 mg/L, respectively. The samples were agitated inside a water bath shaker which is temperature controlled at a speed of 100 rpm at 45 °C. At the expiration of the contact time, suspension of the mixture was filtered, while the remains in the liquid medium was examined for Cd(II) ion using atomic absorption spectrophotometer (AAS) BUCK 211.
Effect of pH on adsorption process
0.2 g of the orange peel was dissolved in 25 mL of 300 mg/L of Cd(II) ion solution in the pH range of 2–9. The pH adjustment to desired value was done by the addition of 0.1 M HCl and/or 0.1 M NaOH prior to the addition of the adsorbent. The flask containing the mixture was equilibrated in an orbital shaker to reach equilibrium at constant agitation speed. The solution was then filtered and Cd(II) concentration left unadsorbed in the solution was investigated using Atomic Absorption Spectrophotometer BUCK 211.
Effect of temperature
In this study, 0.2 g powdered orange peel was agitated with 25 mL of 100 mg/L of Cd(II) ion solution at pH 4.5 and at different temperatures range: 30, 35, 40, 45 and 60 °C. The mixture was shaken constantly with the aid of orbital shaker. The solution was then filtered and the liquid portion was determined using Atomic Absorption Spectrophotometer BUCK 211.
Effect of adsorbent dosage
Impact of dosage of the adsorbent was determined by using various adsorbent dosages from 0.01 to 0.10 g and agitating each of them separately with 25 mL of Cd(II) ion solution at best possible conditions. The mixtures were shaken constantly at the agitation time. Then, the solution was separated and the metal contents of the filtrate were measured using Atomic Absorption Spectrophotometer BUCK 211.
Effect of initial concentration of the Cd(II) ions on adsorption
Exactly 0.1 g of orange peel powder was reacted with 25 mL solution of varying concentration (25–300 mg/L) of the pollutant solution at optimal pH 4.5 over 2 h. The pH was adjusted using 0.1 M HCl and 0.1 M NaOH. The mixtures were shaken constantly with the aid of an orbital shaker for 2 h at 30 °C. The solution was then filtered and the filtrate was measured for Cd(II) ion using Atomic Absorption Spectrophotometer BUCK 211.
Kinetic studies
The kinetics investigation of the uptake of Cd(II) ion in an aqueous solution were done using batch adsorption procedure. In this procedure 0.1 g of sample of OP powder was put in different 200-mL sample tubes and 25 mL of the pollutant was added to each tube. The mixtures were properly agitated for 2 h using a water bath shaker to ensure equilibrium was reached. The content was then separated and the Cd(II) ion remaining in the filtrate was measured using AAS, BUCK 211. The adsorption capacity (mg/g) and removal efficiency (%) were performed using the formulas in Eqs. (1) and (2) as indicated below:
such that Qt (mg/g) signifies the quantity of Cd(II) ions sorbed at t = t; C0 and Ct (mg/L) are the amount of contaminant present at t = 0 and t = t, respectively; the mass of the OP used is given as m (g) and the volume of the pollutant used is denoted as V (L).
Procedure for optimization
Optimization studies of the adsorption of Cd(II) by the orange peel was performed by preparing three different concentrations (50, 150 and 250 mg/L) of Cd(II) ion solutions, solutions were adjusted to pH values of 1.0, 4.5, and 8.0. Different quantities of adsorbent—0.1, 0.055 and 0.010 g were used. A parallel set of experiments without adsorbent served as blank. The flasks containing the mixture (20 mL) each were set into a thermostated shaker at 30 °C and 250 rpm for I h. Each of the flasks was then removed at pre-set time, decanted and the concentration of cadmium ions in the filtrate was determined using AAS BUCK 211.
Reuse study
The reuse study of orange peel was performed using Cd2+ solution. After each adsorption process, the Cd-loaded OP was dried and then dissolved in 100 mL of saturated NaCl solution and stirred for 1 h. During this process, the Cd2+ which adhered to the surface of OP are desorbed due to the fact that the saturated NaCl solution which contains concentrated amount of Na+ are capable of displacing Cd2+ from the OP surface. Thereafter, the cadmium concentrations desorbed were examined using AAS. The Cd-loaded OP was washed, dried and reconstituted for subsequent adsorption tests. Three cycles of adsorption–desorption experiments were done, and the adsorption capacity was calculated after each cycle.
Results and discussion
Characterizations
The diverse functional groups present on orange peel surface prior to and after adsorption were analysed with the aid of FT-IR as shown in Fig. 1. The orange peel demonstrated different functional groups with peak at 3419.82 cm−1 identified as O–H vibrations (Adeogun et al. 2019), the 2926.74 cm−1 peak was recognized as –CH stretching of the alkanes group (Ofudje et al. 2017), 1637.43 cm−1 peak is that of C=O stretching of carbonyl groups, while the one at 1429.33 cm−1 is allotted to –CH3 stretch. The peak noted at 1033.10 cm−1 was allotted to the C–O bond of the esters and carboxylic acids groups (Ofudje et al. 2017). Upon the uptake of cadmium ions, shifts in peak positions were observed indicating the possible involvement of these functional groups. For example, peak at 3419.82 cm−1 increased to 3442.00 cm−1, while peaks at 2926.74 cm−1 and 1637.43 cm−1 increased to 2927.77 and 1642.00 cm−1, respectively. Similarly, the peaks initially present at 1429.33 and 1033.10 cm−1 increased to 1432.21 and 1033.26 cm−1, respectively. Figure 2a depicts the SEM image of the OP powder showing the porous structure which could provide more adsorption sites for the cadmium ions uptake. The XRD patterns of the orange peel adsorbent are shown in Fig. 2b with key diffractions at 2θ = 16.3° and 22.3° which correspond to the (101) and (200) planes, respectively, for cellulose thus indicating an amorphous cellulose type I (Ofudje et al. 2017).
Effect of agitation time and metal concentrations
The role of agitation time (0–240 min) on the removal of Cd(II) ions was carried out with initial Cd(II) ion concentrations within the range of 50–250 mg/L at a solution pH of 5.5 and is as represented in Fig. 3. The maximum uptake of Cd(II) was at 120 min after which there was no considerable increase in the quantity of the pollutant sorbed. As indicated from the plot, the uptake process happens in two steps with the first one being very quick and was attained at about 47 min with percentage removal of 48.4% by orange peel adsorbent. The subsequent stage symbolized a progressive reduced adsorption which was achieved at 120 min. The swift early adsorption could be credited to the growth of Cd(II) ions on to the OP surface, owing to the existence of available cites on the OP surface. Nevertheless, with gradual growth of Cd(II) ions on these spots by the cadmium ions, the uptake became sluggish in the second stage, thus leading to reduction in the amount of pollutant adsorbed by the adsorbent (Adeogun et al. 2018; Ofudje et al. 2020). Also as observed in Fig. 3, when the initial concentration of cadmium ions was increased in the solution, the amount of the metal ions adsorbed onto the adsorbent surface equally increased. This could be as a result of the increase in the number of the molecules of metal ions per unit volume of the medium (Arica et al. 2018).
Effects of pH
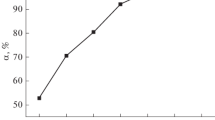
Solution pH has been recognized to be a very important factor in the adsorption procedure since it can affect both the chemistry of the adsorbate and adsorbent. The network of charges of the adsorbate and adsorbent relies on the solution pH. With the rise in the pH from 2 to 5.5, the sorption capacity of OP rose from 24.62 to 44.42% as revealed in Fig. 4. The highest adsorption was accomplished at a solution pH of 5.5. At smaller pH value, the Cd(II) ion elimination is subdued by web of positive charges of the OP and the rivalry that exist between Cd(II) ions and H+ in solution. With elevated pH, the negative charge network on OP increases which is due to the deprotonation of the binding sites. Therefore, the sorption of Cd(II) ions increases (Chunfang et al. 2017; Ofudje et al. 2017). Bayramoglu and Arica (2015) observed that the decrease or increase of biosorption capacity of U(VI) ions at different pH medium can be attributed to the change in surface characteristics of the adsorbent used. It was further noted that the surface characteristics of these adsorbents are composed of different functional groups such as carboxyl, amine, hydroxyl, carbonyl, and phosphate groups and that ion-exchange and electrostatic interactions are mainly responsible for biosorption process. As observed from the FT-IR investigation of OP, the potential functional groups for binding Cd(II) ions are O–H, C=O and C–O groups.
Effect of temperature
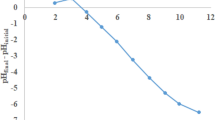
Figure 5 demonstrates the dependence of the adsorption of Cd(II) by orange peel on the temperature. The adsorption process was found to be a function of temperature and the highest Cd(II) uptake was achieved at 45 °C. The rise of solution temperature increased the adsorption competence of Cd(II) on OP indicating that the process was endothermic. Increase in temperature increased the rate of the progression of the Cd(II) ions from the solution onto the unoccupied sites of OP and weakened the thickness of the surface layer of OP, thereby enhancing the dispersion resistance of adsorbates to adsorbents (Ofudje et al. 2020).
Effect of dosage
The percentage removal of Cd(II) on OP significantly increased with the adjustment of OP from 0.01 to 0.04 g as depicted in Fig. 6 which showed that adsorption of Cd(II) ion was enhanced as the OP dosage increases. This is owing to more active spots which are accessible for the adsorption of Cd(II) ion due to lager surface area provided by increase in OP concentration. The common patterns of enhanced pollutant adsorption with a rise in sorbent dosage signify the existence of more binding sites which are available for adsorption. Arica et al. (2018) observed that an increase in the adsorbent dosage provides increase in the available sites on the adsorbent with for the uptake of the adsorbate, and this lead to an increase in the removal efficiency of adsorbent.
Kinetic studies
Plots of \(Q_{t}\) against t were used to determine kinetic values of pseudo-first-order, pseudo-second-order, Elovich and intraparticle diffusion models which are presented in Eqs. 3 to 6, respectively (Lagergren 1898; Ho and McKay 1998; Cheung et al. 2001; Weber and Morris, 1963):
Given that \(k_{1}\) (min−1g) and \(k_{2}\) (min−1g/mg) are the rate constants for first- and second-orders, respectively, \(Q_{e}\) (mg/g) signifies the quantity of Cd(II) ions adsorbed at equilibrium and Qt is as defined previously.
Test of kinetic fitness
The best fit among the kinetics models was tested by the sum of error squares (SSE, %) given by (Adeogun et al. 2013; Ofudje et al. 2017):
such that N represents the data points of number.
The estimated values of \(Q_{e}\), \(k_{1}\) and \(k_{2}\) were calculated from the non-linear plots of \(Q_{t}\) against t in Fig. 7 are as presented in Table 1. The R2 values obtained from the pseudo-first-order equation ranged from 0.964 to 0.995, whilst that of the second-order equation ranged from 0.988 to 0.997 with the pseudo-second-order showing better values. However, a close inspection at the values of Qeexp and Qecal of the second-order showed greater differences and this suggested that the second-order equation cannot be useful to pseudo-describe the uptake process. On the other hand, careful inspection of values of the Qecal from the first-order model correspond well with the Qeexp; which indicates that the pseudo-first-order model can be used to explain the whole adsorption process of Cd(II) ion onto the surface of OP which is known as physisorption. Investigation of sum of error squares (SSE, %) further affirm the suitability with the pseudo-first-order model when compared with that of second-order model. The rate constant values from the pseudo-first-order model range from 0.076 to 0.134 min−1. From the Elovich model, α represents the initial rate of adsorption which is measured in mg/g min, while the desorption constant in g/mg is given as β which were derived from the plots of \(Q_{t}\) against t from least square fit method as shown in Fig. 7c. Also, Kid stands for the intraparticle diffusion rate constant measured in mgg−1 min−0.5 and Ci stands for the degree of surface thickness. The values of R2 as obtained from the intraparticle diffusion model are in the range of 0.957 to 0.980 thus, suggesting the applicability of this model in describing the adsorption pattern of the biomass. The uptake process of cadmium ions can be categorized into three steps: (i) film mass transfer, (ii) intraparticle diffusion, and (iii) chemical reaction on adsorbent (Ofudje et al. 2017). The preliminary rapid uptake of Cd(II) ion by OP is as a result of the abundant presence of adsorptive sites, greater outer diffusion rate of Cd(II) ions couple with small diameter of Cd(II) ions. However, reduction in adsorption was due to the transfer of Cd(II) ions from external diffusion to internal, while saturated adsorption is as a result of the repulsion between pollutant and sorbent which hindered the activities of the remaining active sites.
Equilibrium studies
To investigate the equilibrium behaviour of orange peel powder, Langmuir, Dubinin–Radushkevich (D-R), and Freundlich isotherm were utilized. Langmuir isotherm assumed that the adsorption energy is the same irrespective of the amount of adsorbed material on the adsorbent active sports which can be described by the relationship in Eq. 8 (Langmuir 1918; Dehghani et al. 2015 and Morovat et al. 2016):
with \(Q_{\hbox{max} }\) standing for the maximum monolayer adsorption capacity (mg/g), while b (L/mg) denotes the adsorption energy. The Langmuir separation factor (RL) can be expressed as:
such that Co (mg L−1) gives the adsorbate concentration and when the values of RL lies between 0 and 1, it implies favourable adsorption, if RL > 1, it indicates unfavourable adsorption and if RL = 0, it means irreversible process.
Freundlich isotherm described a multisite or multilayer with a heterogeneous surface and is given according to Eq. 10 below (Freundlich 1906):
where KF (mg/g) (mg/L)−1/2 is the sorption capacity and n is the intensity of adsorption of the adsorbent, respectively. The Dubinin–Radushkevich (D–R) adsorption isotherm is applicable at low concentration range of adsorbate, which could be useful in providing explanations regarding homogeneous and heterogeneous adsorption surfaces. The non-linear representation of the D–R equation is given as (Dubinin et al. 1947):
with Qm representing the saturation adsorption capacity of the OP, value of β stands for the adsorption free energy and ε is the potential for Polanyi and can be expressed as:
The ideal molar gas constant is given as R (8.31 J/mol K) and T is temperature (K). Value of the mean sorption energy, E (kJ/mol), could be estimated from β parameter as thus:
Values of E (kJ/mol) could be used to predict the mechanism of adsorption. For instance, when E values lie between 8 and 16 kJ/mol, it is an ion-exchange process, but values of E less than 8 kJ/mol suggest physical adsorption process while values of E greater than 16 kJ/mol, imply that the adsorption process is subjugated by particle diffusion. The plots of the isotherms are as shown in Fig. 8, while their values are as given in Table 2. The values of R2 gotten from Langmuir, Freundlich and D–R isotherms for Cd(II) ions are 0.995, 0.996 and 0.993, respectively, thus suggesting that the three isothermal models could be employed to describe the equilibrium adsorption data, particularly the Langmuir and Freundlich models. The maximum adsorption capacity Qm and KF obtained for Cd(II) ions uptake are 128.23 (mg g_1) and 54.531 (mg/g)(mg/L)−1/2 in that order. The n values represent the distribution energy of adsorption site. Since n > 1, it indicates feasible adsorption, with greater heterogeneous adsorption site and energy distribution. Value of RL obtained is < 1, which implies that the adsorption was favourable. Dubinin–Radushkevich (D–R) parameter, E, was found to be 0.757 kJmol−1 and since the value of E is smaller than 8 kJ mol−1, the adsorption mechanism is physical in nature. Table 3 indicates the potentials of various adsorbents for cadmium ion adsorption compared with orange peel powder from this present study. The result demonstrates better adsorption capacity of orange peel adsorbent compared to tea-industry waste and olive cake.
Thermodynamic studies
The thermodynamic investigation of the adsorption of cadmium onto orange peel was evaluated using the relations:
with R standing for the universal gas constant (8.314 J mol−1K−1) and T is the absolute temperature in Kelvin. The free energy change is given as ΔG◦, enthalpy change is ΔH◦ and the entropy change as ΔS◦). The plots of InKd versus 1/T should give a slope of ΔHo/R with an intercept of ΔSo/R as shown in Fig. 9 and the evaluated parameters are as presented in Table 4. Positive value of enthalpy change affirmed the adsorption process to be endothermic, which support the claim early that the process of adsorption is physical in nature. Furthermore, the negative value of the entropy change showed that the degree of randomness decreased as the temperature increased and shows a decreased disorderliness at the solid/solution interface.
Optimization studies
The mutual relationship of pH and adsorbent dosage on the elimination of Cd by orange peel is represented as contour and 3D plots in Fig. 10a and it clearly depicts that the uptake of Cd(II) by orange peel depends on the solution pH, which was observed to be increasing (till pH of 5.0) and thus optimum adsorption took place at pH 5.5. According to Dehghani et al, (2016), decrease in Cd(II) sorption was notable as the solution pH was adjusted from 5.5 to 8. The adsorbent dosage also has a synergistic effect on the percentage removal. The joint effects of sorbent dosage as well as that of Cd(II) ion concentration on the efficiency of adsorption behaviour of OP in the uptake of Cd are presented in Fig. 10b. The removal efficiency rose with the sorbent dosage from 0.02 to 0.10 g. With greater sorbent dosage, more binding spots become accessible to the Cd(II) on the surface of the sorbent material, which leads to greater removal capability. It was noted that the optimum removal efficiency of 60% was accomplished at 0.10 g of the adsorbent dosage. In general, greater sorbent dosage and lesser Cd(II) ion concentration enhanced the uptake of Cd (II) ions (Dehghani et al. 2016). The impact of solution pH on the uptake of Cd(II) ion was also studied in the pH range of 1–8 as presented in Fig. 10c. The results showed that as the pH decreases, the concentration of Cd(II) ion adsorbed increases.
Reuse study
The reusability of the adsorbent in adsorption study is one of the major advantages of considering this method over conventional methods for wastewater treatment. To this end, reuse study was conducted following the reaction of the used OP with solution of saturated NaCl solution for 1 h as depicted in Fig. 11. It was observed that as the number of reuse increases, the adsorption capacity of the OP decreases. Though the adsorption capacity of the tested adsorbent for cadmium decreases with subsequent adsorption process, this decrease was minimal thus suggesting the reusability of OP for more than one cycle. The adsorption capacity decreased from 88.34 to 73.42%.
Conclusion
This study examined the potentials of waste agricultural products of orange peel as less-expensive and environmentally benign adsorbent in the subtraction of cadmium ions from aqueous solution under diverse experimental circumstances. The result from the equilibrium study gave the adsorption capacity of 128.23 mg g−1 at 318 K, while the three isotherm models tested conform well with the contaminant experimental data, although Langmuir demonstrated a better fitting. The kinetic data aligned perfectly with the pseudo-first-order equation. Thermodynamic analysis showed that the adsorption process was endothermic in nature and this is an indication of good economic value. Result from reuse study showed that the adsorbent demonstrated good potentials for regeneration. Thus, orange peel could be a useful adsorbent for uptake of cadmium ions in a contaminated environment.
Availability of data and materials
Not applicable
Abbreviations
- AAS:
-
Atomic absorption spectrophotometer
- a T :
-
Tempkin constants relating to binding constant (Lmg−1)
- b :
-
Langmuir equilibrium constant (L mg−1)
- b T :
-
Heat of adsorption
- β :
-
Mean free energy of adsorption
- C e :
-
Equilibrium concentrations
- C o :
-
Initial concentrations
- C i :
-
Degree of surface thickness
- E :
-
Mean free energy (kJ mol−1)
- EDX:
-
Energy dispersive X-ray analysis
- D–R:
-
Dubinin–Radushkevich
- FT-IR:
-
Fourier-transform infrared spectroscopy
- K F :
-
Freundlich adsorption capacity
- K id :
-
Intraparticle diffusion rate constant measured in mgg−1 min−0.5
- K d :
-
Equilibrium constant
- m :
-
Adsorbent mass (g)
- n :
-
Adsorbent intensity
- Q e :
-
Amount of adsorbate adsorbed in mg/g
- Q max :
-
Maximum amount of solute adsorbed
- k 1 :
-
Adsorption rate constant (min−1) for a Pseudo first-order
- k 2 :
-
Rate constant of pseudo-second-order (g mg−1 min−1)
- ε :
-
Polanyi potential
- OP:
-
Orange peel
- N :
-
Data points of number
- R :
-
Molar gas constant (8.314 J mol−1 K−1)
- R 2 :
-
Correlation coefficient
- R L :
-
Separation factor
- SEM:
-
Scanning electron microscope
- SSE:
-
Sum of square error function
- T:
-
Temperature (K)
- V:
-
Volume (L) of the pollutant taken
- XRD:
-
X-ray diffractometer
- ΔSo :
-
Entropy change
- ΔHo :
-
Enthalpy change
- ΔGo :
-
Free energy change
References
Adeogun AI, Idowu MA, Ofudje EA, Kareem SO, Ahmed SA (2013) Comparative biosorption of Mn(II) and Pb(II) ions on raw and oxalic acid modified maize husk: kinetic, thermodynamic and isothermal studies. Appl Water Sci 3:167–179
Adeogun AI, Ofudje EA, Idowu MA, Kareem SO, Vahidhabanu S, Babu BR (2018) Biosorption of Cd2+ and Zn2+ from aqueous solution using tilapia fish scale (Oreochromis sp): kinetics, isothermal and thermodynamic study. Desalin Water Treatment 107:182–194
Adeogun AI, Akande JA, Idowu MA, Kareem SO (2019) Magnetic tuned sorghum husk biosorbent for effective removal of cationic dyes from aqueous solution: isotherm, kinetics, thermodynamics and optimization studies. Appl Water Sci 9:160. https://doi.org/10.1007/s13201-019-1037-2
Arica A, Kuman M, Gercel O, Ayas E (2018) Poly(dopamine) grafted bio-silica composite with tetraethylenepentamine ligands for enhanced adsorption of pollutants. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2018.11.003
Bayramoglu G, Arica MY (2015) Amidoxime functionalized Trametes trogii pellets for removal of uranium(VI) from aqueous medium. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-015-4224-0
Baysal Z, Cinar E, Bulut Y, Alkan H, Dogru M (2009) Equilibrium and thermodynamic studies on biosorption of Pb(II) onto Candida albicans biomass. J Hazard Mater 161:62–67
Bernard A (2008) Cadmium & its adverse effects on human health. Indian J Med Res 128(4):557–564
Brinza L, Nygård CA, Dring MJ, Gavrilescu M, Benning LG (2009) Cadmium tolerance and adsorption by the marine brown alga Fucus vesiculosus from the Irish Sea and the Bothnian Sea. Bioresour Technol 100:1727–1733
Cay S, Uyanık A, Özaşık A (2004) Single and binary component adsorption of copper(II) and cadmium(II) from aqueous solutions using tea-industry waste. Sep Purif Technol 38:273–280
Cheung CW, Porter JF, McKay G (2001) Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Resour 35:605–612
Chunfang T, Yan S, Riqing Z, Xin L, Jinfeng SL, Yuting Z, Danling O (2017) Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv. 7:16092–16103
Dehghani MH, Mahvi AH, Rastkari N, Saeedi R, Nazmara S, Iravani E (2015) Adsorption of bisphenol A (BPA) from aqueous solutions by carbon nanotubes: kinetic and equilibrium studies. Desalin Water Treat. 54:84–92
Dehghani HM, Zarei A, Mesdaghinia A, Nabizadeh R, Alimohammadi M, Afsharnia M (2016) Response surface modeling, isotherm, thermodynamic and optimization study of arsenic (V) removal from aqueous solutions using modified bentonite-chitosan (MBC). Korean J Chem Eng 32:1–11
Doyurum S, Celik A (2006) Pb(II) and Cd(II) removal from aqueous solutions by olive cake. J Hazard Mater B138:22–28
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons. I. Adsorption of organic vapors. Zh Fiz Khim 21:1351–1362
Freundlich HMF (1906) Über die adsorption in lösungen, Z. Phys Chem 57:385–470
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4:219–232
Gavrilescu M, Pavel LV, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163:475–510
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. J Environ Sci Health B 76(4):183–191
Ho YS, Chiu WT, Hsu CS, Huang CT (2004) Sorption of lead ions from aqueous solution using tree fern as a sorbent. Hydrometallurgy 73:55–61
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104
Lagergren S (1898) About the theory of so-called adsorption of soluble substance. Kung Sven Veten Hand 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lei T, Sheng-Jian L, Jiang F, Zi-Xuan R, Li-Lian W, Xiang-Jun Y, Li-Hong T, Shi-Xiong W (2019) Adsorption of cadmium ions from an aqueous solution on a highly stable dopamine-modified magnetic nano-adsorbent. Nanoscale Res Lett 14:352. https://doi.org/10.1186/s11671-019-3154-0
Min-Suk K, Jeong-Gyu K (2020) Adsorption characteristics of spent Co_ee grounds as an alternative adsorbent for cadmium in solution. Environments. https://doi.org/10.3390/environments7040024
Mohan D, Singh KP (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse-an agricultural waste. Water Res 36:2304–2318
Mohan D, Pittman CU Jr, Steele PH (2006) Single, binary and multicomponent adsorption of copper and cadmium from aqueous solutions on Kraft lignin-a biosorbent. J Colloid Interf Sci. 297:489–504
Morovat M, Chamani M, Zarei A, Sadeghi A (2016) Dietary but not in ovo feeding of Silybum marianum extract resulted in an improvement in performance, immunity and carcass characteristics and decreased the adverse effects of high temperatures in broilers. Br Poult Sci 57:105–113
Ofudje EA, Williams OD, Asogwa KK, Awotula AO (2013) Assessment of Langmuir, Freundlich and Dubinin–Radushkevich Adsorption Isotherms in the study of the biosorption of Mn(II) ions from aqueous solution by untreated and acid-treated corn shaft. Int J Sci Eng Res 4(7):1628–1634
Ofudje EA, Awotula AO, Hambate GV, Akinwunmi F, Alayande SO, Olukanni OD (2017) Acid activation of groundnut husk for copper adsorption: kinetics and equilibrium studies. Desalin Water Treatment 86:240–251
Ofudje EA, Adeogun AI, Idowu MA, Kareem SO, Ndukwe NA (2020) Simultaneous removals of cadmium (II) ions and reactive yellow 4 dye from aqueous solution by bone meal derived apatite: kinetics, equilibrium and thermodynamic evaluations. J Anal Sci Technol. 11:7. https://doi.org/10.1186/s40543-020-0206-0
Peijia L, Jiajia W, Junmo A, Jaeheon L (2019) Adsorption characteristics of Cd(II) and Ni(II) from aqueous solution using succinylated hay. Int J Miner Metall Mater 26:1239–1246. https://doi.org/10.1007/s12613-019-1832-7
Pintilie S, Branza L, Betianu C, Pavel LV, Ungureanu F, Gavrilescu M (2007) Modelling and simulation of heavy metals transport in water and sediments. Environ Eng Manag J 6:153–161
Ramdani A, Kadeche A, Adjdir M, Taleb Z, Ikhou D, Taleb S, Deratani A (2020) Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract Technol. 15:1. https://doi.org/10.2166/wpt.2020.003
Riaz U, Murtaza G, Saifullah Farooq M (2018) Comparable effect of commercial composts on chemical properties of sandy clay loam soil and accumulation of trace elements in soil plant system. Int J Agric Biol 20:85–92. https://doi.org/10.17957/IJAB/15.0433
Sadrzadeh M, Mohammadi T, Ivakpour J, Kasiri N (2008) Separation of lead ions from wastewater using electrodialysis: comparing mathematical and neural network modeling. Chem Eng J 144 (3):431–441
Saeed A, Iqbal M, Akhtar MW (2005) Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater B117:65–73
Wang X, Cui Y, Peng Q, Fan C, Zhang Z, Zhang X (2020) Removal of Cd(II) and Cu(II) from aqueous solution by Na+-modified pisha sandstone. J Chem. https://doi.org/10.1155/2020/2805479
Weber WJ, Morris JC (1963) Kinetic of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Acknowledgements
The support of all the technical staff of CSIR-CECRI, India, in the characterization of the sample is acknowledged.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have equal contribution to this research work. All authors read and approved the final manuscript.
Authors’ information
Dr. T.F. Akinhanmi is a Senior Lecturer at the Department of Chemistry, College of Physical Sciences, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria. Her area of specialization is Analytical Chemistry. Dr. E.A. Ofudje is currently a Senior Lecturer at the Department of Chemical Sciences, College of Basic and Applied Sciences, Mountain Top University, Ogun State, Nigeria. His area of specialization includes Physical/Environmental/Material Chemistry. Dr. A.I. Adeogun is an Associate Professor in the Department of Chemistry, College of Physical Sciences, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria. He specialized in Physical and Environmental Chemistry. Peter Aina and Joseph Ilo Mayowa are both students in the Department of Chemistry, College of Physical Sciences, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors do declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akinhanmi, T.F., Ofudje, E.A., Adeogun, A.I. et al. Orange peel as low-cost adsorbent in the elimination of Cd(II) ion: kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 7, 34 (2020). https://doi.org/10.1186/s40643-020-00320-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-020-00320-y