Abstract
The purpose of this study was to investigate whether different surface treatments, executed before and after the sintering process, affect the surface characteristics (roughness and morphology) and shear bond strength of computer-aided design–computer-aided manufacturing (CAD–CAM) milled zirconia luted with self-adhesive resin cement onto human dentin. Sixty-four rectangular zirconia slabs (6 × 8.5 × 2.3 mm thickness) were prepared using the CAD–CAM system and randomly distributed into two groups according to the moment of treatment, before and after sintering, and four subgroups according to the surface treatment (n = 8): C (untreated; control), AO (aluminum oxide air abrasion), GB (glass beads air abrasion), and HA (etching with hydrofluoric acid). The samples were evaluated using a confocal laser microscope to assess the surface roughness and morphology. For the shear bond strength test, the milled zirconia was cemented onto dentin slabs using self-adhesive resin cement and submitted to the test in a universal testing machine. Data were submitted to a two-way ANOVA and Tukey test (p < 0.05). The surface roughness was always higher for the samples treated before sintering (highest roughness in the GB group). When treatment was employed after sintering, there was no difference between the subgroups. The shear bond strength analysis showed that the adhesion was greater when the treatment was employed after sintering, where groups AO, HA, and GB showed similar bond strength, higher than group C. Thus, it is concluded that regardless of the treatment performed, the bond strength is greater when zirconia is treated after sintering, while the surface roughness is greater before sintering.
Similar content being viewed by others
Introduction
The use of zirconia dioxide-based ceramic in dentistry was first reported by Meyenberg in 1995 [1]. It offers a wide range of applications such as intra-root retainers for endodontic-treated teeth, implants, implant abutments and infrastructure, crowns, and fixed partial dentures [2,3,4,5]. Among its advantages are superior mechanical properties, low thermal conductivity, aesthetics, color stability, and excellent biocompatibility [6, 7].
Zirconia is a polymorphic material, which has three allotropic phases: monoclinic, tetragonal, and cubic [6]. To obtain the zirconia as a coherent solid, the sintering process is performed, which consists of a thermal treatment in which the crystal structure of zirconium oxide changes [8, 9], turning from the monoclinic to the tetragonal or the cubic phase [10]. When stimuli are applied to sintered zirconia, it might still respond through transformation-toughening mechanisms (tetragonal to monoclinic phase transformations), which is the main reason for the superiority of the mechanical properties in comparison to other ceramic materials available [8,9,10,11,12]. Basically, as grains in a monoclinic crystallographic arrangement present higher volume in comparison to the tetragonal arrangement, they lead to introduction of residual compressive stress, which in the presence of cracks actuates difficulting its propagation [11, 12].
Despite the superior mechanical performance, one of the main problems of zirconia is related to adhesion failure as a result of its polycrystalline microstructure (absence of a glassy matrix) [13, 14], which is due to the fact that the mechanism to achieve enhanced mechanical interlocking is based on hydrofluoric acid etching, promoting dissolution of a glassy matrix on vitreous ceramic [9, 13, 15]. Thus, pursuit of techniques to improve bonding to the zirconia-based ceramic systems is important to prevent infiltration and increase fracture resistance [15]. Efforts to modify the characteristics of the surface energy of this material have been proposed by several manufacturers and researchers by employing different surface treatments [10, 16,17,18,19], such as sandblasting with aluminum oxide [20], blasting with glass beads [13, 18], coating with silica [21], hydrofluoric acid etching [21, 22], milling, diamond abrasion, and others [15]. It is important to highlight that there is also a relationship between surface treatments and phase transformation [9, 10].
In this sense, the same phase transformation mechanism responsible for mechanical enhancement is also responsible for zirconia deterioration through a low-temperature degradation mechanism [6, 11]. Basically, as the monoclinic phase content increases and surpasses a critical percentage content (studies suggest a percentage superior to 50%) [11], it results in increased surface roughness, porosity (decrease in material density), and, finally, compromising the mechanical properties of the ceramic. Therefore, finding approaches to achieving enhanced adhesion to zirconia without triggering extensive phase transformation is important.
Usually, the treatment of the ceramic surface is performed after the sintering process. However, Moon suggested that blasting performed before sintering could lead to a final proportion of favorable tetragonal structure [23], which could have a positive effect on the clinical performance of zirconia restorations, preventing the use of an already transformed material in response to the surface treatment to enhance bonding during luting zirconia restorations. Therefore, much effort should be focused on executing studies that evaluate and compare the performance of zirconia ceramic to treatments executed in different stages of its processing steps (before and after sintering) to fully understand and characterize these mechanisms.
Thus, the aim of this study is to investigate whether different surface treatments, executed before and after the sintering process, affect the surface characteristics (roughness and morphology) and shear bond strength of computer-aided design–computer-aided manufacturing (CAD–CAM) milled zirconia luted with self-adhesive resin cement onto human dentin.
Materials and methods
The experimental protocol was approved by the ethical committee on human research of the School of Dentistry of Ribeirão Preto, University of São Paulo (Process No. 59993416.8.0000.5419).
Experimental design
This study presented a factorial design, with the factor moment of treatment at two levels (before or after sintering) and surface treatment at four levels (subgroups): C (control; no surface treatment), AO (aluminum oxide air abrasion), GB (glass beads air abrasion), and HA (etching with a hydrofluoric acid solution). Sixty-four zirconia pre-sintered slabs were randomly assigned into these eight experimental subgroups (n = 8). The quantitative response variables were shear bond strength testing and analyses of zirconia surface roughness measured by confocal laser microscopy. The qualitative response variable was the analyses of zirconia surface morphology.
Zirconia specimen preparation
Pre-sintered zirconium dioxide (ZrO2) (Ceramill Zi 71 XS 12 mm, Amann Girrbach AG, Koblach, Austria) rectangular ceramic specimens (6 × 8.5 × 2.3 mm thickness) were prepared according to the CAD–CAM (Ceramill, Amann Girrbach AG, Koblach, Austria) technique following manufacturer’s instructions. Prior to surface treatments, all specimens were ultrasonically cleaned (Biosonic UC 50; Coltene Whaledent, Cuyahoga Falls, OH) for three cycles of 1 min each in distilled water to remove surface residue, left on a paper towel, and gently air dried until completely dry.
Zirconia surface treatments
- Subgroup C::
-
No extra surface treatment was performed on the specimens of the control group.
- Subgroup AO::
-
The zirconia specimens were grit-blasted with irregular 50 μm aluminum oxide particles (Cobra, Renfert, Germany) at a constant pressure of 2.0 bar for 10 s using sandblasting equipment (Vario Basic Jet Blaster, Renfert, Hilzingen, Germany). The direction of sandblasting was perpendicular to the zirconia surface, and the working distance was 10 mm [24]. The specimens were then rinsed thoroughly with water by means of a triple syringe for 30 s.
- Subgroup GB::
-
The rectangular zirconia specimens were grit-blasted with regular 50 μm glass microbeads (Rolloblast, Renfert, Germany) using the same protocol employed for the AO group.
- Subgroup HA::
-
Zirconia specimens were etched with 10% hydrofluoric acid solution (Condac porcelana, FGM Produtos Odontologicos, Joinville, SC, Brazil) for 30 s. The specimens were rinsed as described for the previous groups.
Evaluation of zirconia surface roughness and morphology by confocal laser microscopy
The zirconia surface was evaluated before and after the sintering process. A 3D laser confocal microscope (LEXT, Olympus Corporation, Japan) and OLS4000 software (LEXT, Olympus Corporation, Japan) were used to obtain images of all zirconia specimens. For the evaluation of surface roughness, representative images were obtained with 107× amplification and analyzed by computer software built into the microscopy setup. Surface roughness linearly in the central area (2574 × 2577 μm) of each specimen was measured (Sa—according to ISO 25178-2:2012) [25], and the mean value of each group was calculated. For the qualitative morphology analyses, a scan of the entire surface of each specimen was performed, and images of the most representative areas of the specimen were obtained using 4262× amplification.
Zirconia sintering process
After evaluation of the surface roughness of the pre-sintering zirconia groups (n = 32) by confocal laser microscopy, all rectangular ceramic specimens (n = 64) were fully sintered in a furnace using a sintering program of unitary elements (Ceramill Therm, Amann Girrbach AG, Koblach, Austria) at a heating rate of 8 °C/min to 1450 °C for 120 min followed by a 10-h cooling phase [26]. The group treated after sintering (n = 32) was then distributed into four subgroups and submitted to the four different surface treatments following the same protocol previously described.
Dentin sample preparation
Sixty-four recently extracted and non-carious mandibular human third molars were randomly selected for this study. They were stored in 0.1% thymol solution at 4 °C until use. Teeth were further washed in running water for 24 h to eliminate thymol residues. Crowns were sectioned on the cemento-enamel junction with a precision saw machine (Isomet 1000, Buehler, Lake Forest, USA) under refrigeration. Each crown was further fixed on plexiglass plates and sectioned in occlusal, mesial, distal, and cervical orientation. The enamel surface of the fragments was polished with a polishing machine (Arotec APL-4, Arotec, São Paulo, SP, Brazil) under cooling with a #1200 grit paper (Norton Abrasives Ltda, São Paulo, SP, Brazil) until the enamel was completely removed from the surface. Thus, 64 rectangular dentin samples 6 mm wide, 8.5 mm in height, and 2 mm thick were obtained.
The slabs were embedded in self-cure acrylic resin (Jet Classic, São Paulo, SP, Brazil) surrounded by a polyvinyl chloride (PVC) cylinder (1.5 cm in diameter and 1.5 cm high) with the vestibular dentin surface facing up. After resin polymerization, the PVC cylinders were removed and the specimens were stored at 37 °C in distilled water for 24 h. After this period, the dentin surfaces were polished with #400 and #600 grit silicon carbide (SiC) paper (3 M, Sumaré, Brazil), and additional grinding was accomplished with #1200 grit SiC paper for 1 min to produce a standardized smear layer, under cooling, using a polishing machine. Specimens were stored at 37 °C in 95% relative humidity.
Adhesion of zirconia on dentin
The zirconia specimens were cemented to the dentin slabs using Panavia (Panavia F 2.0, Kuraray Co Ltd, Osaka, Japan) resin cement. The cementation technique application was performed according to the manufacturer’s instructions. First, the dentin surfaces were cleaned with fluoride-free pumice, rinsed with water, and dried gently with a triple syringe. One drop of each ED Primer II Liquid A was mixed with Liquid B, applied to the dentin tooth surface, and left for 30 s. Then, the dentin was dried with a gentle air stream until the surface appeared glossy. The A paste and B paste of Panavia F 2.0 cement were dispensed in equal amounts and mixed for 20 s. A thin and even layer of the resin cement was then applied to the zirconia pretreated surface. The zirconia specimens were set over the dentin samples and held in place under a load of 1 kgf. Excess resin cement was removed with a hand instrument, and the dentin/zirconia set was light cured (DB 686, Dabi Atlante, Ribeirão Preto, SP, Brazil—800 mW cm2) for 20 s in each direction (zirconia top and then at bonding interface at 0°, 90°, 180°, and 270°). Specimens were stored in distilled water at 37 °C for 24 h.
Shear bond strength testing
Shear bond strength was tested with a universal testing machine (Instron Model 2519-106, Instron Corporation, Canton, MA, USA) at a crosshead speed of 0.5 mm/min using a chisel loading applicator carefully positioned perpendicular to the bond interface and as close as possible to the interfacial region. A shear load was applied until failure occurred. The bond strength (s) values, expressed in MPa, were calculated using the following formula: \({\text{Stress}}\, = \,{\text{Failure load }}\left( {\text{N}} \right)/{\text{surface area }}({\text{mm}}^{ 2} )\). The bonded surface area of the specimens was approximately 51 mm2 according the specimen dimensions (6 mm wide, 8.5 mm height, and 2 mm thick). The analyses were carried out for all 64 specimens. A fractography analysis of all failed specimens was conducted using a stereomicroscope.
Data analysis
The data obtained were subjected to normality tests (Shapiro–Wilk) and homogeneity of variance (Levene). Data of the shear bond strength and surface roughness were normal and homogeneously distributed and were submitted to parametric analysis (two-way ANOVA). For all the analyses, multiple comparisons were performed using the Tukey test. The probability level was 95% in all analyses. For qualitative analysis of zirconia surface morphology, images of representative areas were evaluated and visually compared.
Results
Evaluation of zirconia surface roughness by confocal laser microscopy
The surface roughness was higher for the zirconia treated before sintering, regardless of the treatment performed (p < 0.05). When treatment was employed after sintering, there was no difference between the subgroups (p > 0.05). Conversely, when the treatment was employed before sintering, the GB group (6.55 µm ± 0.39) presented higher roughness (p < 0.05) (Table 1).
Confocal laser microscopy qualitative analysis
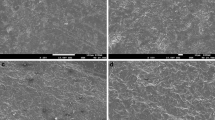
Confocal laser microscopy images qualitatively showed that before sintering, the glass beads air abrasion resulted in a rougher surface compared to the other treatments, with the presence of depictable localized defects. The C group presented a surface that appeared more homogeneous. The other two groups, HA and AO, qualitatively showed intermediary patterns (Fig. 1). After sintering, all treatments employed resulted in similar roughness surface and pattern, and when compared with the treatments employed before sintering, the surface appeared less rough, regardless of the treatment performed (Fig. 1).
Shear bond strength testing
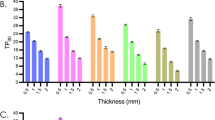
The shear bond strength analysis showed that the adhesion was greater when the treatment was employed after sintering, regardless of the zirconia protocol treatment employed (p < 0.05). There was no interaction between the moment of treatment and surface treatment type factors (p > 0.05). Regarding the treatments employed for both treatment times considered (before and after sintering process), the C group always presented the lowest bond strength values (p < 0.05). Aluminum oxide air abrasion and glass beads air abrasion treatments led to higher bond strength values, statistically similar to that observed for the HA group (although the last one presented a noticeable tendency for decreased values, without reaching statistically significant intensity) (Table 2; Fig. 2). All tested specimens presented adhesive failure patterns (cement-zirconia interface), and no pretest failures were noticed.
Discussion
In the present study, it was investigated whether different treatments, before and after sintering, affect surface roughness, morphology, and the shear bond strength of dual-cured resin cement (Panavia F) to the zirconia surface. It was clearly shown that if the treatment is executed before sintering, the surface becomes rougher and the bond strength is not optimized.
In recent years, many studies have been executed in an attempt to create a predictable and long-lasting result of enhanced adhesion to zirconia ceramic. Even systematic reviews have been published on this aim [27,28,29]. However, it is important to highlight that few studies have considered an already milled ceramic surface, and fewer have considered a tri-layer bonding assembly (ceramic, cement, and dentin). In this sense, until now, the best data available point to air abrasion with different particles as the most relevant treatment [27,28,29]. Our data are in accordance with previous data as the air-abraded groups presented the highest bond strength values when executed after sintering, but without statistical difference.
Air abrasion after sintering of zirconia promotes enhanced micromechanical interlocking by surface alterations and increased roughness [27, 30]. Our data pointed out that when a complex assembly that involved clinical aspects such as the milling of the ceramic (a substrate that is already rough) and the tri-layer assembly of a ceramic specimen being luted with resin cement to a dentin substrate, no protocol resulted in high bonding values (the highest values of bond strength were below 4 MPa). Indeed, the bond strength values observed by us are smaller than typically noticed elsewhere, which are commonly reported as approximately 15–20 MPa [27, 28]. Therefore, there are clear indications that adherence zirconia ceramic should still be considered as challenging, and efforts are still needed to develop and optimize treatment options to this aim.
When the air abrasion treatment was employed before sintering, the roughness was high, so it probably blocked the flow of resin cement and the fill-up of the surface irregularities, which increased the chance of air bubbles being present. This could be the reason for the lowest performance on bond strength in comparison to treatments after sintering and is in agreement with previous findings [10]. In contrast, other researchers reported no significant differences in the shear bond strength values between the groups abraded before or after zirconia sintering [21, 31]. The different results shown in the literature can be explained by the lack of standardization between cementation protocols used in the studies because some used silane or adhesive monomers on the zirconia surface after sintering, which improved the wettability of zirconia by the cement, resulting in greater adhesion [10, 32,33,34].
Another aspect of the treatments executed previous to sintering is that they lead to higher surface roughness, regardless of the treatment performed. This can be explained by the fact that before sintering, the material is fragile; thus, any treatment has the potential to be harmful. This was also shown in a previous study [35]. Concerning the different air abrasion treatments, the use of glass beads created higher surface roughness than oxide aluminum. This finding can be explained by the different irregularity of the particles because aluminum oxide particles are irregular and glass beads are microspheres. Thus, studies evaluating the format and physical–chemical characteristics of the particles could clarify this issue.
After sintering, neither surface treatment altered the superficial roughness, indicating adequate performance without introducing harmful defects. Therefore, in this condition, the milled surface roughness generated by the CAD–CAM system was not increased by air abrasion; on the contrary, there was a tendency to decrease roughness (without reaching a statistical level) explained by a probable wear of higher topographical aspects of the surface morphology leading to a more homogeneous surface.
Another important aspect of adhesion to zirconia highlighted in the literature is the use of luting systems containing phosphate monomer (MDP) [29, 36]. Kern reviewed and compared the best available clinical and laboratory evidence for successful bonding of dental oxide ceramic restorations and concluded that the association of air abrasion at a moderate pressure with MDP-containing primers and/or resin cements provides long-term durable bonding to zirconia ceramic [37]. However, our data are based on the use of an MDP-containing resin cement, and even then, as previously mentioned, high bond strength values were not noticed.
Because this study adopted an in vitro experimental design, it presents inherent limitations. The absence of an energy-dispersive X-ray spectroscopy analysis under scanning electron microscopy could be considered a limitation of this study because it could have been a powerful tool to elucidate the ceramic surface chemical interactions (alterations) promoted by surface treatments and the influence of sintering on those characteristics. Also, the absence of aging and the choice of a macro shear test assembly could be considered limitations. Nevertheless, further studies should be conducted to evaluate new treatment methods for better bond strength results.
Conclusion
Within the methods and results of the present study, it is concluded that regardless of the treatment performed on the surface of the zirconia manufactured by a CAD–CAM system, the bond strength is greater when the treatment is employed after the sintering process, despite the lower surface roughness.
Abbreviations
- CAD–CAM:
-
computer aided design–computer aided machining
- C:
-
control
- AO:
-
aluminum oxide
- GB:
-
glass beads
- HA:
-
hydrofluoric acid
- ZrO2 :
-
zirconium dioxide
- 3D:
-
tridimensional
- PVC:
-
polyvinyl chloride
- SiC:
-
silicon carbide
- MPa:
-
megapascal
- N:
-
Newton
- MDP:
-
phosphate monomer
- EDS:
-
energy-dispersive X-ray spectroscopy
References
Meyenberg KH, Luthy H, Schärer P. Zirconia posts: a new allceramic concept for nonvital abutment teeth. J Esthet Dent. 1995;7:73–80.
Chen Q, Baino F, Pugno NM, Vitale-Brovarone C. Bonding strength of glass-ceramic trabecular-like coatings to ceramic substrates for prosthetic applications. Mater Sci Eng C Mater Biol Appl. 2013;33:1530–8. https://doi.org/10.1016/j.msec.2012.12.058.
Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007;35:819–26.
Passia N, Mitsias M, Lehmann F, Kern M. Bond strength of a new generation of universal bonding systems to zirconia ceramic. J Mech Behav Biomed Mater. 2016;62:268–74. https://doi.org/10.1016/j.jmbbm.2016.04.045.
Sentuerk U, Von Roth P, Perka C. Ceramic on ceramic arthroplasty of the hip: new materials confirm appropriate use in young patients. Bone Joint J. 2016;98(1 Suppl A):14–7. https://doi.org/10.1302/0301-620x.98b1.36347.
Chevalier J, Gremillard L, Deville S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu Rev Mater Res. 2007;37:1–32. https://doi.org/10.1146/annurev.matsci.37.052506.084250.
Steyern P, Carlson P, Nilner K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. J Oral Rehabil. 2005;32:180–7. https://doi.org/10.1111/j.1365-2842.2004.01437.x.
Fonseca RG, Abi-Rached FO, Reis JMSN, Rambaldi E, Baldissara P. Effect of particle size on the flexural strength and phase transformation of an airborne-particle abraded yttria-stabilized tetragonal zirconia polycrystal ceramic. J Prosthet Dent. 2013;110:510–4. https://doi.org/10.1016/j.prosdent.2013.07.007.
Souza ROA, Valandro LF, Melo RM, Machado JP, Bottino MA, Ozcan M. Air-particle abrasion on zirconia ceramic using different protocols: effects on biaxial flexural strength after cyclic loading, phase transformation and surface topography. J Mech Behav Biomed Mater. 2013;26:155–63. https://doi.org/10.1016/j.jmbbm.2013.04.018.
Abi-Rached FO, Martins SB, Almeida-Júnior AA, Adabo GL, Góes MS, Fonseca RG. Air abrasion before and/or after zirconia sintering: surface characterization, flexural strength, and resin cement bond strength. Oper Dent. 2015;40:E66–75. https://doi.org/10.2341/14-013-LR1.
Pereira GKR, Venturini AB, Silvestri T, Dapieve KS, Montagner AF, Soares FZM, Valandro LF. Low-temperature degradation of Y-TZP ceramics: a systematic review and meta-analysis. J Mech Behav Biomed Mater. 2016;55:151–63. https://doi.org/10.1016/j.jmbbm.2015.10.017.
Hjerppe J, Närhi TO, Vallittu PK, Lassila LV. Surface roughness and the flexural and bend strength of zirconia after different surface treatments. J Prosthet Dent. 2016;116:577–83. https://doi.org/10.1016/j.prosdent.2016.02.018.
Blatz MB, Chiche G, Holst S, Sadan A. Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int. 2007;38:745–53.
Sailer I, Makarov NA, Thoma DS, Zwahlen M, Pjetursson BE. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: single crowns (SCs). Dent Mater. 2015;31:603–23. https://doi.org/10.1016/j.dental.2015.02.011.
Thompson JY, Stoner BR, Piascik JR, Smith R. Adhesion/cementation to zirconia and other non-silicate ceramics: where are we now? Dent Mater. 2011;27:71–82. https://doi.org/10.1016/j.dental.2010.10.022.
Akin GE, Kaval ME, Turk T, Akin H. Surface roughness and bond strength of zirconia posts to a resin cement after various surface pretreatments. Photomed Laser Surg. 2015;33:246–51. https://doi.org/10.1089/pho.2014.3861.
Atsu SS, Kilicarslan MA, Kucukesmen HC, Aka PS. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J Prosthet Dent. 2006;95:430–6.
Cavalcanti AN, Foxton RM, Watson TF, Oliveira MT, Giannini M, Marchi GM. Bond strength of resin cements to a zirconia ceramic with different surface treatments. Oper Dent. 2009;34:280–7. https://doi.org/10.2341/08-80.
Wolfart M, Lehmann F, Wolfart S, Kern M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater. 2007;23:45–50.
Borges GA, Sophr AM, de Goes MF, Sobrinho LC, Chan DC. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J Prosthet Dent. 2003;89:479–88.
Menani LR, Farhat IA, Tiossi R, Ribeiro RF, Guastaldi AC. Effect of surface treatment on the bond strength between yttria partially stabilized zirconia ceramics and resin cement. J Prosthet Dent. 2014;112:357–64. https://doi.org/10.1016/j.prosdent.2013.08.025.
Casucci A, Osorio E, Osorio R, Monticelli F, Toledano M, Mazzitelli C, Ferrari M. Influence of different surface treatments on surface zirconia frameworks. J Dent. 2009;37:891–7.
Moon JE, Kim SH, Lee JB, Ha SR, Choi YS. The effect of preparation order on the crystal structure of yttria-stabilized tetragonal zirconia polycrystal and the shear bond strength of dental resin cements. Dent Mater. 2011;27:651–63. https://doi.org/10.1016/j.dental.2011.03.005.
Pozzobon JL, Pereira GKR, Wandscher VF, Dorneles LS, Valandro LF. Mechanical behavior of yttria-stabilized tetragonal zirconia polycrystalline ceramic after different zirconia surface treatments. Mater Sci Eng C Mater Biol Appl. 2017;77:828–35.
ISO 25178-2 (2012) Geometrical product specifications (GPS)—surface texture: Areal—Part 2: terms, definitions and surface texture parameters. International Organization for Standardization.
Karl M, Graef F, Wichmann M, Krafft T. Passivity of fit of CAD/CAM and copy-milled frameworks, veneered frameworks, and anatomically contoured, zirconia ceramic, implant-supported fixed prostheses. J Prosthet Dent. 2012;107:232–8.
Ozcan M, Bernasconi M. Adhesion to zirconia used for dental restorations: a systematic review and meta-analysis. J Adhes Dent. 2015;17:7–26. https://doi.org/10.3290/j.jad.a33525.
Tzanakakis EG, Tzoutzas IG, Koidis PT. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J Prosthet Dent. 2016;115:9–19. https://doi.org/10.1016/j.prosdent.2015.09.008.
Thammajaruk P, Inokoshi M, Chong S, Guazzato M. Bonding of composite cements to zirconia: a systematic review and meta-analysis of in vitro studies. J Mech Behave Biomed Mater. 2018;80:258–68. https://doi.org/10.1016/j.jmbbm.2018.02.008.
Inokoshi M, De Munck J, Minakuchi S, Van Meerbeek B. Meta-analysis of bonding effectiveness to zirconia ceramics. J Dent Res. 2014;93:329–34. https://doi.org/10.1177/0022034514524228.
Turp V, Sen D, Tuncelli B, Goller G, Özcan M. Evaluation of air-particle abrasion of Y-TZP with different particles using microstructural analysis. Aust Dent J. 2013;58:183–91. https://doi.org/10.1111/adj.12065.
Curtis AR, Wright AJ, Fleming GJ. The influence of surface modification techniques on the performance of a Y-TZP dental ceramic. J Dent. 2006;34:195–206. https://doi.org/10.1016/j.jdent.2005.06.006.
Monaco C, Cardelli P, Scotti R, Valandro LF. Pilot evaluation of four experimental conditioning treatments to improve the bond strength between resin cement and Y-TZP ceramic. J Prosthodont. 2011;20:97–100. https://doi.org/10.1111/j.1532-849X.2010.00677.x.
Wang RR, Lu CL, Wang G, Zhang DS. Influence of cyclic loading on the fracture toughness and load bearing capacities of all-ceramic crowns. Int J Oral Sci. 2014;6:99–104. https://doi.org/10.1038/ijos.2013.94.
Monaco C, Tucci A, Esposito L, Scotti R. Microstructural changes produced by abrading Y-TZP in presintered and sintered conditions. J Dent. 2013;41:121–6. https://doi.org/10.1016/j.jdent.2012.06.009.
Matinlinna JP, Ozcan M, Lassila LV, Vallittu PK. The effect of a 3-methacryloxypropyltrimethoxysilane and vinyltriisopropoxysilane blend and tris (3-trimethoxysilylpropyl) isocyanurate on the shear bond strength of composite resin to titanium metal. Dent Mater. 2004;20:804–13.
Kern M. Bonding to oxide ceramics—laboratory testing versus clinical outcome. Dent Mater. 2015;31:8–14. https://doi.org/10.1016/j.dental.2014.06.007.
Authors’ contributions
FCL—prepared the zirconia specimens; RGPD—co-orientation of the study and evaluated the surface roughness and morphology by confocal laser microscopy; LBC—performed the adhesion of the zirconia on dentin; RFR—performed the shear bond strength testing; VAC—performed the zirconia sintering process; EAG—performed the statistical analysis; review of the manuscript; GKRP—was a major contributor in writing the manuscript; AOS—was a major contributor in writing the manuscript; MDSN—orientation of the study and review of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to greatly thank Doctor Fernando Lopes and Gabriel Silva Tomazela for their help and support during laboratory procedures in Digital Center laboratory.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
Authors highlight that they do not have any funding.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lopes, F.C., Palma-Dibb, R.G., Campi, L.B. et al. Surface topography and bond strength of CAD–CAM milled zirconia ceramic luted onto human dentin: effect of surface treatments before and after sintering. Appl Adhes Sci 6, 8 (2018). https://doi.org/10.1186/s40563-018-0110-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40563-018-0110-7