Abstract
Background
The drug-metal complexes are used to control the growth of pathogens and parasites which are harmful to humans. Moxifloxacin (MOX) is a new fourth generation 8- methoxy fluoroquinolone. Its chemical name is [1-cyclopropyl-7-(S,S)-2,8-diazabicyclo(4.3.0)- non-8-yl-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinoline carboxylic acid hydrochloride]. Many researchers have reported Moxifloxacin-metal complexes with transition metals and studied their biological applications. We have synthesized novel moxifloxacin- Au(III) and Ag(I) metal complexes ([Au(MOX) 2 (Cl) 2 ].2H 2 O and [Ag (MOX) 2 ].2H 2 O), characterized, and found their biological activities.
Methods
Moxifloxacin- Au(III) and Ag(I) metal complexes were prepared by adding corresponding aqueous solutions of Au(III) and Ag(I) metal salts to methanolic solution of Moxifloxacin. These metal complexes were characterized by physio-chemical techniques like UV-Vis, 1 H- NMR, FTIR, XRD, DSC, TGA, SEM and microanalytical data. The disc diffusion method was used to study the antibacterial activity of the Moxifloxacin-metal complexes.
Results
The structural assessment of these complexes has been carried out based on physio- chemical and spectroscopic methods. The powder X-ray diffraction data of the metal complexes revealed moderate crystallinity. SEM analysis confirms that [Ag(MOX) 2 ].2H 2 O complex has small rods like morphology whereas [Au(MOX) 2 (Cl) 2 ].2H 2 O has well crystalline rods like morphology. The results from DSC of moxifloxacin- Au(III) and Ag(I) metal complexes revealed the interaction between the drug and metals. Further, Au & Ag moxifloxacin metal complexes have shown significant antibacterial activity against gram-positive and gram-negative bacteria.
Conclusions
The spectral and analytical results clearly confirmed the coordination chemistry of Au (III) and Ag (I) Moxifloxacin metal complexes. The IR, electronic transition and elemental data led to the conclusion that the geometry of the complex of Au(III) is octahedral and that of Ag (I) complex is tetrahedral. The [Ag(MOX) 2 ].2H 2 O complex has showed good antibacterial activity compared to [Au (MOX) 2 (Cl) 2 ].2H 2 O metal complex. The antibacterial activity studies indicate the metal complexes have more biological activity than free ligand.
Similar content being viewed by others
Background
Nowadays, most of the metal ions play an important role in many biological processes like biological function of proteins, and operating many regulation, stabilization, completion courses of cellular functions. The use of gold metal in complexation with drugs in modern, twentieth century medicine began with the discovery in 1890 by the German bacteriologist Robert Koch that gold cyanide K [Au (CN)4] was bacteriostatic towards the tubercle bacillus. In 1920s, gold therapy was introduced for tuberculosis. Gold drugs have been used to treat a variety of diseases like psoriatic arthritis, a form of arthritis associated with psoriasis, juvenile arthritis, palindromic rheumatism, and discoid lupus erythematosus. Encouraging results have also been obtained with gold therapy as a treatment for various inflammatory skin disorders such as pemphigus, urticaria, and psoriasis (Thomas and Papandrea 1993).
Silver compounds have been used in external preparations as an antiseptic, including both silver nitrate and silver proteinate, which can be used in dilute solution as eye drops to prevent conjunctivitis in newborn babies. Silver nitrate is also sometimes used in dermatology in solid stick form as a caustic (“lunar caustic”) to treat certain skin conditions, such as corns and warts. Additionally, silver nitrate is used in certain laboratory settings to stain cells (Lansdown 2006; Kokura et al. 2010). Silver has also been used in cosmetics, intended to enhance antimicrobial effects and the preservation of ingredients.
Researchers had a significant interest in the utilization of metal complexes as drugs to treat several human diseases. Metal and metal-drug complexes medicinal uses and their applications are increasing clinically for commercial importance. The preparation and characterization of the drug-metal complexes and evaluation (Sadeek et al. 2011; Power and Phillips 1993) of the biological activity have gained significance. According to chelation theory, chelation enhances the biological activity of metal complexes by increasing the complexity. Preparation of drug-metal complexes is of most significance in current days for curing of diseases by inhibition of growth of pathogens. It is clear from the recent literature that the trend towards the preparation of the drug-metal complexes has huge applications and will continue to occur in future medicinal applications (Ott 2009; Gasser et al. 2011; Maftei et al. 2015; Simon et al. 2005). However, the newly prepared drug-metal complexes have been more significant in present days, because they show good biological activity and these metal complexes find a role in detecting diseases (Neda et al. 2000; Filimon et al. 2010). The drug-metal complexes were also used to control the growth of pathogens and parasites which are harmful to humans.
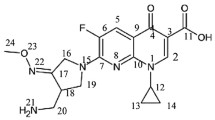
Moxifloxacin (MOX) is a new fourth generation 8-methoxy fluoroquinolone (Fig. 1). Its chemical name is [1-cyclopropyl-7-(S,S)-2,8-diazabicyclo(4.3.0)-non-8-yl-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinoline carboxylic acid hydrochloride]. The drug was developed mainly for the treatment of community-acquired pneumonia and upper respiratory tract infections. Moxifloxacin is also used for the treatment of hospital-acquired infections and has a very good safety and tolerability profile with trade names Avelox, Avalox, and Avelon. Moxifloxacin is to be considered a drug of the last remedy when all other drugs are failed. It inhibits DNA gyrase, a topoisomerase IV, and type II topoisomerase.
The investigators are well-known with the preparation of different metal complexes of moxifloxacin. W.F. El-Hawary et al. have reported spectrophotometric determination and biological activity of moxifloxacin.HCl. Sadeek et al. gave detailed study about the preparation and characterization of moxifloxacin metal complexes and their biological applications. Amina et al. have reported metal complexes of moxifloxacin–imidazole mixed ligands: characterization and biological studies. The majority of researchers reported about the preparation and characterization of the moxifloxacin-metal complexes with transition metals and studied their biological applications (Nakamoto et al. 1968; Soayed et al. 2013; Sultana et al. 2014).
However, reported literature has limited characteristics and investigations in the moxifloxacin-metal complexes. These metal complexes were shown moderate activity against gram-positive and negative bacteria. To our knowledge, no report is available in the literature about the synthesis and characterization of noble metal complexes of moxifloxacin. The development of noble metal-based drugs with promising pharmacological application may offer unique therapeutic opportunities (Rafique et al. 2010). The inorganic chemistry is provided with better opportunities to use the noble metal complexes (Mihorianu et al. 2016; Maftei et al. 2016a) as therapeutic agents on living organisms which is different from transition metals and non-metals. The noble metal-drug complexes have significant applications (Maftei et al. 2016b; Neda et al. 1993a) in medicine and show a great diversity in action.
We report here synthesis and characterization of novel moxifloxacin–Ag(I) and Au(III) metal complexes, [Au(MOX)2(Cl)2].2H2O and [Ag (MOX)2].2H2O. The synthesized moxifloxacin metal complexes were characterized by physio-chemical methods like UV-Vis, H1-NMR, FTIR, XRD, DSC, TGA, and SEM. Antibacterial activity studies were carried out against gram-positive and gram-negative bacteria for the prepared moxifloxacin metal complexes.
Methods
Materials
Moxifloxacin and gold(III) chloride were purchased from the Sigma-Aldrich Limited, India. Silver nitrate, methanol, and ether were purchased from MERK, India. All the chemicals and reagents are analytical reagent grade and were used without further purification. All glassware and magnetic stir bars were cleaned in an aquaregia solution (3HCl: HNO3) and then cleaned with double distilled (DD) water.
Procedure for synthesis of Au (III) moxifloxacin metal complex
It was prepared by adding 1.6 g (0.01 M) amount of moxifloxacin ligand in 30 ml of methanol to the Au (III) ion (0.005 M) in distilled water and stirred about 12 h at 50 °C and cooled to room temperature. Yellow color precipitate of the metal complex was obtained with good yield. The precipitate was filtered and washed with hot water followed by cold methanol to free from unreacted metal salts and ligand. Finally, the residue was washed with ether and dried in a desiccator.
Moxifloxacin HCl
IR (KBr, cm−1): 3530 (O–H stretching of HOH; COOH), 3156 (aromatic C–H stretching), 2978, 2800 (aliphatic C–H stretchings), 1709 (C=O stretching; COOH), 1620 (N–H bending), 1592, 1533 (aromatic C–C stretching), 1490 (C=C stretching), 1422, 1323 (aliphatic C–H bending), 1245 (C–F stretching), 1184 (C–O stretching), 1166 (C–N stretching), 1111 (C–C stretching), 1016, 991 (C–H bending; phenyl). 1H NMR (DMSO) ppm: δ 10.87 (s,2H;NH2+), 9.03 (s,1H;COOH), 8.78 (s,1H;Ar–CH–), 7.81, 7.77 (s,1H, Ar–CH), 4.16, 4.14 (2H,H–2′,–CH2), 4.07 (t,2H,H–4′,N–CH2), δ3.85 (s,3H,–OCH3), δ 3.72 (m,2H,H–3′,–CH2), δ3.34 (t, 2H,H–1′,N–CH2), δ 2.98 (s,1H, H–6′,N–CH–, diasterotopic), δ2.73(s,1H, H–7′,–CH–,diasteriotopic), 1.358 (t,1H,H–8′,–CH–), 1.088 (t,2H,H–9′,–CH2), 0.934 (t,2H,H–10′–CH2).
Moxifloxacin–Au(III) complex
Yield: 75%; IR (KBr, cm−1): 3375 (O–H stretching), 3056 (aromatic C–H stretching), 2955, 2885 (aliphatic C–H stretchings), 1620 (N–H bending), 1525 (C=C stretching;phenyl), 1462 (aromatic C=C stretching), 1420, 1321 (aliphatic C–H bending), 1369 (N–H bending), 1325 (C–F stretching), 1189 (C–O stretching), 1080 (C–N stretching), 1049 (C–C stretching), 1031, 975 (C–H bending;phenyl).
1H NMR (DMSO) δ ppm: 10.54 (s,2H;NH2+), 9.07 (s,1H;COOH), 8.78 (s,1H;Ar–CH–), 7.81, 7.77 (s,1H, Ar–CH), 4.16, 4.14 (2H,H–2′,–CH2), 4.07 (t,2H,H–4′,N–CH2), 3.85 (s,3H,–OCH3), 3.72 (m,2H,H–3′,–CH2), 3.34 (t, 2H,H–1′,N–CH2), 2.98 (s,1H, H–6′,N–CH–,diasterotopic), 2.73 (s,1H,H–7′,–CH–,diasteriotopic), 1.35 (t,1H,H–8′,–CH–), 1.08 (t,2H,H–9′,–CH2), 0.93 (t,2H,H–10′–CH2).
Procedure for synthesis of Ag (I) moxifloxacin metal complex
It was prepared by adding required 1.6 g (0.01 M) amount of moxifloxacin ligand in 30 ml of methanol to the Ag (I) ion (0.005 M) in distilled water and stirred about 8 h. Light yellow color precipitate of the metal complex was obtained in good yield. The precipitate was filtered and washed with hot water followed by cold methanol to free from unreacted metal salts and ligand and finally with ether and dried in a desiccator.
Moxifloxacin–Ag(I) complex
Yield: 80%; IR (KBr, cm−1): 3380 (O–H stretching), 3046 (aromatic C–H stretching), 2953, 2880 (aliphatic C–H stretchings), 1624 (N–H bending), 1516 (C=C stretching; phenyl), 1459 (aromatic C=C stretching), 1422, 1323 (aliphatic C–H bending), 1376 (N–H bending), 1320 (C–F stretching), 1185 (C–O stretching), 1084 (C–N stretching), 1051 (C–C stretching), 1030, 965, 877 (C–H bending;phenyl). 1H NMR (DMSO) δ ppm: 10.38 (s,2H;NH2+), 9.06 (s,1H;COOH), 8.78 (s,1H;Ar–CH–), 7.80, 7.76 (s,1H, Ar–CH), 4.16, 4.14 (2H,H–2′,–CH2), 4.07 (t,2H,H–4′,N–CH2), 3.85 (s,3H,–OCH3), 3.72 (m,2H,H–3′,–CH2), 3.34 (t, 2H,H–1′,N–CH2), 2.93 (s,1H, H–6′,N–CH–,diasterotopic), 2.73 (s,1H, H–7′,–CH–, diasteriotopic), 1.35 (t,1H,H–8′,–CH–), 1.08 (t,2H,H–9′,–CH2), 0.93 (t,2H,H–10′–CH2).
Characterization
The characterization of the synthesized moxifloxacin ligand and its metal complexes were recorded with UV-Vis-NIR spectrophotometer (UV-3600, Schimadzu) at 200–700 nm. FTIR analysis was carried out by KBr pellet method using JASCO FTIR 4600 in the scanning range of 450–4000 cm−1 used for recording spectrum. The powder XRD technique was used to study crystalline nature of the AuNPs by XRD (Rigaku, Miniflex) method with Cu kα (λ = 1.5418 Å) radiation. The morphology of the prepared moxifloxacin and its metal complexes was examined by SEM. The 1H NMR spectra of the ligand and its metal complexes were recorded on an AV-400 MHz NMR spectrometer in IICT, Hyderabad in DMSO-d6 and CDCl3 solvents at room temperature. Microanalysis data were obtained using a Carlo Erba EA1108 elemental analyzer. Thermograms were obtained using Perkin Elmer DSC 4000 differential scanning colorimeter at Vishnu Chemical, Hyderabad, India. Melting points were determined on the Kofler Hotstage microscope (Reichart Thermovar) and were uncorrected.
Biological activity studies
The disc diffusion method was used to study the antibacterial activity of moxifloxacin, and its gold(III) and silver(I) metal complexes. B.Cereus, Pseudomonas Auruginosa, B. Subtilis, and E. coli were used as model test strains. Prepared Luria-Bertani (LB) agar medium was transferred into sterilized Petri dishes and then solidified. Petri plates were spread with bacterial strains in a laminar airflow hood. Using micropipette, 10, 20, 30, and 40 μL of the AuNPs solution and 10 μL of streptomycin solutions were added to each well of all plates. They were dried in the laminar hood and incubated 24 h at 37 °C. All bacterial zones of inhibition were measured and assay was performed in triplicate.
Results and discussion
Electronic spectra and magnetic measurements
UV-Visible absorption spectral data of the moxifloxacin ligand and its moxifloxacin–Au(III) and moxifloxacin–Ag(I) metal complexes were shown in Table 1. The electronic transition of the ligand occurred at 292 nm (Table 3). But on complexation with the different metal ions like Au (III) and Ag (I), new bands appeared at 299 and 295 nm, respectively corresponding to the transitional charge transfer from the ligand to the different metal ions. Bands occurred in the region of 295 and 298 nm, respectively for [Ag(MOX)2]·2H2O and [Au(MOX)2(Cl2)].2H2O metal complexes (Sultana et al. 2014; Kondaiah et al. 2017) are assigned to charge transfer transition (L → M). The magnetic moments of the present moxifloxacin of Au (III) and Ag (I) complexes are 1.80 and 1.75 BM, respectively. The magnetic moments of these ranges are suggested octahedral geometry for Au (III) and tetrahedral geometry for Ag (I) complex (Kadyrov et al. 1996; Neda et al. 1996; Sonnenburg et al. 1994).
FTIR spectroscopic studies
The IR spectra of moxifloxacin ligand and its Au (III) and Ag(I) metal complexes were recorded by FTIR spectrophotometer (Kunze et al. 2002; Maftei et al. 2013; Neda et al. 1993b; Plinta et al. 1994). The FTIR spectra of the silver and gold complexes of moxifloxacin ligand exhibited major changes as compared to the free ligand. The strong bands at 1707 and 1624 cm−1 in the spectrum of moxifloxacin are assigned pyridone carbonyl and carboxyl stretches. In the FTIR spectra of Au (III) and Ag(I) complexes, the band at 1707 cm−1 was not recorded due to the formation of a bond between the metal ion and carboxylate oxygen (Fig. 2.) Two new bands are appeared for these complexes (Tulkens et al. 2012; Muhammad et al. 2007), at 1624 and 1335 cm−1, 1620 and 1329 cm−1, were assigned to asymmetric and symmetric ʋ(COO), respectively, for Ag (I) and Au (III)–moxifloxacin metal complexes. The difference values [DV = ʋas(COO)–ʋs(COO)] of 289 and 290 for Au(III) and Ag (I) complexes respectively are used as an evidence for the bidentate chelation mode of the group. These results revealed that the chelation of metals on moxifloxacin in this work.
1H NMR spectroscopic studies
The proton NMR spectra of moxifloxacin Ag(I) and Au(III) metal complexes have been recorded in CDCl3 and DMSO–d6 and compared to the spectrum of moxifloxacin (Fig. 3). The moxifloxacin ligand 1H NMR spectra, a broad singlet signal, were observed at δ 10.87 ppm corresponding to (2H,NH2+), and a strong singlet signal appeared at δ 9.03 ppm corresponding to –COOH. The aromatic protons that are H-2 and H-5 are very close to the coordination site of the moxifloxacin ligand. H-2 proton of moxifloxacin ligand appeared, strong singlet at δ 8.78 ppm and H-5 proton of moxifloxacin ligand noticed at (δ 7.81, 7.77 ppm) and appeared as a doublet. A triplet at (δ 4.18, 4.16, 4.14 ppm) due to [2H,N–4′–CH2]. The H-1′and H-5′ protons were observed as doublet signal at (δ 4.07, 4.02 ppm), i.e., [4H,(2N–CH2)], a singlet of 3H of OCH3 group appeared at δ 3.85 ppm. The spectral band of [1H,8′–H] was shown at δ 3.30 ppm, a triplet at [4H (δ 2.98, 2.91, 2.73 ppm)] due to [4H (2H-2′, 2H-3′)] and (δ 1.28, 1.29, 1.35 and 0.93, 0.87 ppm)] due to [4H, (2H-9′, 2H-10′)]. Upon addition of a metal, these protons undergo the most significant changes. In the metal complexes, the H-2 proton gives a new signals at δ = 8.72 and 8.62 ppm while H-5 proton signals were appeared at δ 7.76 and 7.81 ppm, respectively, for Au (III) and Ag (II) moxifloxacin metal complexes (Figs. 4 and 5) (Chiririwa and Muzenda 2014; Ali et al. 2013; Efthimiadou et al. 2006). This shift is due to the complexation and difference in the configuration of complexes than ligand. In the 1H NMR spectrum, a signal observed at δ 9.03 ppm due to carboxylic –OH proton in the ligand is shifted to δ 9.11 and 9.07 ppm respectively for Au (III) and Ag (II) moxifloxacin metal complexes.
Powder XRD studies of moxifloxacin metal complexes
Powder XRD studies of moxifloxacin–Ag (I) metal complex
The XRD patterns are used to explain qualitatively the degree of crystallinity. The diffractogram (6 diffractions) reflects between 20 and 80° (2θ) values (Fig. 6) for moxifloxacin–Ag (I) complex. All the peaks have been indexed 2θ values compared in the graph. On comparison, the values reveal that there is a good agreement (Arayne et al. 2005; Turel 2002) between values of 2θ and d values. The diffraction peaks observed at 2θ values 26.71, 27.91, 32.22, 46.25, 54.84, and 57.53° were indexed as (110), (110), (111), (002), (211), (013), and 113, respectively, as shown in Fig. 6. The maximum orientation observed at (002) at 2θ of 32.22 °. The formation of [Ag(MOX)2].2H2O metal complex was confirmed by X-ray diffraction technique. These results agreed well with the standards of the Joint Committee on powder diffraction standards (JCPDS NO.01-072-0607). The X-ray diffraction data of MOX–Ag (I) complex are presented in Table 2. The powder X-ray diffraction data showed identical features with moderate crystallinity (Drevensˇek et al. 2006).
Powder XRD studies of moxifloxacin–Au (III) metal complex
The XRD patterns are used to explain qualitatively the degree of crystallinity. The diffractogram (6 diffractions) reflects between 20 and 80° (2θ) values (Fig. 7) for moxifloxacin–Au (III) complex. All the peaks have been indexed 2θ values compared in the graph. On comparison, the values reveal that there is good agreement between values of 2θ and d values. The diffraction peaks observed at 2θ values 24.44, 28.601, 32.52, 46.43,54.13, 57.53, and 78.48 ° were indexed as (101), (111), (002), (211), (103), (113), and (322), respectively, as shown in the Fig. 7. The formation of [Au(MOX)2(Cl2)].2H2O metal complex was confirmed by X-ray diffraction technique (De Almeida et al. 2007). These results are in good agreement with the standards of the Joint Committee on powder diffraction standards (JCPDS no. 04-0784). The X-ray diffraction data of MOX–Au(III) complex are presented in Table 3. The powder X-ray diffraction data showed moderate crystallinity.
SEM analysis
The SEM micrographs (morphology) of the moxifloxacin HCl have been taken in different magnification. The moxifloxacin HCl showed small needle-like morphology. [Ag(MOX)2].2H2O complex has shown small rods like structure whereas [Au(MOX)2(Cl)2].2H2O showed well crystalline rod-like (Efthimiadou et al. 2008) structures (Fig. 8). The SEM micrographs of moxifloxacin metal complexes, [Au(MOX)2(Cl)2].2H2O and [Ag(MOX)2].2H2O, reveal the structural modification, and it clearly indicates the formation of complexes.
Differential scanning colorimeter
DSC thermograms have shown an endothermic peak of moxifloxacin pure drug at 238.35 °C, which corresponded to its melting point. DSC thermograms (Figs. 9 and 10) of moxifloxacin–Au(III) and moxifloxacin–Ag(I) metal complex showed endothermic peaks at 261 and 228.67 °C, respectively (Khairnar and Singh 2016). The results of the thermograms obtained from DSC of moxifloxacin–Au(III) and Ag(I) metal complexes revealed the interaction between the drug and metals.
Thermal behavior of Au (III) and Ag (II) moxifloxacin metal complexes
The thermogravimetric studies of all the complexes were carried out in the air at a heating rate of 10 °C per minute. The thermoanalytical data are summarized in Table 4. The thermal decomposition of the complexes proceeds in three stages. Au (III) and Ag (I) complexes are thermally stable up to 132 and 138 °C, respectively. This table agreed with the elemental analysis of Table 5. The first stage of decomposition corresponds to endothermic dehydration of complexes by the loss of two water molecules and chlorine occurs in the temperature range 145–320 and 140–325°C, respectively (Attimarad et al. 2012; Drevensˇek et al. 2006). The second decomposition with exothermic peak by the loss of ligand moiety occurs in the temperature at 490 and 492 °C, respectively. The solid residues above 530 and 500 °C were identified as Au and Ag metal oxides, respectively. In all the complexes, the final products are metal oxides (Chawla et al. 2012; Trindade et al. 2006).
The molar conductance of complexes in DMF (~ 10–3 M) was determined at 27 + 2 °C using systronic 303 reading conductivity bridge for Au(III) and Ag (I) complexes of moxifloxacin. These complexes are highly soluble in dimethylformamide (DMF). Therefore, these metal chelates are dissolved in DMF to perform conductivity measurements (El-Megharbel et al. 2015; Kljun et al. 2011; Khairnar and Singh 2016). A known amount of solid complex was transferred into 25 ml standard flask and dissolved in DMF. The contents were made up to the mark with DMF. The complex solution is transferred into a clean and dry 100 ml beaker. The molar conductance values of these metal complexes are measured and found to be 4.30 and 4.40 for Au (III) and Ag (II) complexes, respectively. These values suggest non-electrolytic nature (Gobec et al. 2014) of the present complexes.
The elemental analysis of moxifloxacin ligand and its metal complexes also performed; the compositions of the metal ligands with percentages calculated and found were tabulated in Table 5. The colored solid MOX–HCl complexes were stable in the air and insoluble in H2O and most organic solvents except for DMSO and DMF upon gentle heating (Patel et al. 2011). The products were determined as non-electrolytes. The structures of MOX–HCl complexes were deduced as [Au(MOX)2(Cl)2].2H2O and [Ag(MOX)2].2H2O.
Antibacterial activity
The antibacterial activity of the moxifloxacin drug and its metal complexes (as a standard moxifloxacin) was tested against bacteria because bacteria can achieve resistance to antibiotics through biochemical and morphological modifications (Sultana et al. 2014; Mihorianu et al. 2016; Maftei et al. 2016a; Maftei et al. 2016b). The organisms used in the present investigations included Staphylococcus aureus (S. aureus), Streptococcus features (S. features) and Bacillus subtillis (B. subtilis) (as gram-positive bacteria) and Proteus mirabilis (P. mirabilis), Pseudomonas aereuguinosa (P. aereuguinosa), and Escherichia coli (E. coli) (as gram-negative bacteria). The zone of inhibition of moxifloxacin and its metal complexes against gram-negative and gram-positive bacteria were then evaluated by the well diffusion method (De Almeida et al. 2007; Efthimiadou et al. 2008; Patel et al. 2011; Singh et al. 2013; Tavares et al. 2004). The results of the bacterial screening of the synthesized compounds were recorded as shown in Table 6. The bacterial inhibition clear zones of both gram-positive and gram-negative bacteria can be observed around the well, while the zone of inhibition of moxifloxacin was moderate compared to all the metal complexes. Antibacterial activity of all the metal complexes significantly increased compared to the standard moxifloxacin. In these, moxifloxacin–Ag (I) metal complexes showed increased antibacterial activity compared to Au–moxifloxacin metal complexes (Nabd El-Wahed et al. 2009).
Conclusions
In this research work, novel Au(III) and Ag (I) moxifloxacin metal complexes were synthesized and characterized by magnetic susceptibility, elemental analysis, UV-Vis, FTIR, 1H-NMR, TGA, DSC, and SEM. The spectral and analytical results clearly described the coordination chemistry of Au (III) and Ag (I) moxifloxacin metal complexes. The IR, electronic transition, and elemental data lead to the conclusion that the geometry of the complexes of Au(III) is octahedral and that of Ag (I) complex is tetrahedral structure. Hence, the structures of moxifloxacin metal complexes are given in Fig. 11. In all the complexes, the ligand acts as a bidentate. These metal complexes have shown significant antibacterial against the tested organisms. The [Ag (MOX)2].2H2O complex showed good antibacterial activity compared to [Au (MOX)2(Cl)2].2H2O metal complex. The antibacterial activity indicates the metal complexes have more biological activity than free ligands. All the metal chelates are found to be non-electrolytes. These results will definitely encourage more research towards drug-metal complexes in medicine.
Abbreviations
- [Ag(MOX)2]·2H2O (II):
-
Moxifloxacin–Ag(I)metal complex
- [Au (MOX)2(Cl2)].2H2O (I):
-
Moxifloxacin–Au(III)metal complex
- 1H-NMR:
-
Proton nuclear magnetic resonance spectroscopy
- DSC:
-
Differential scanning colorimeter
- FTIR:
-
Fourier transform infrared spectroscopy
- MOX–HCl:
-
Moxifloxacin hydrochloride
- TGA:
-
Thermo gravimetric analysis
- UV-Vis:
-
Ultra violet visible spectroscopy
- XRD:
-
X-ray diffraction studies
- AAS:
-
Antibacterial activity studies
- MAD:
-
Micro analytical data
References
Ali KA, Abd-Elzaher MM, Mahmoud K. Synthesis and anticancer properties of silver(I) complexes containing 2,6-Bis(substituted)pyridine derivatives. Int J Med Chem. 2013; https://doi.org/10.1155/2013/256836.
Arayne MS, Sultana N, Hussain F. Interaction between ciprofloxacin and antacids. Adsorption and dissolution studies. Drug Met & Drug Inter. 2005;21:117–29.
Attimarad M, Al-Dhubiab BE, Alhaider IA, Nair AB, Sree Harsha N, Mueen Ahmed K. Simultaneous determination of moxifloxacin and cefixime by first and ratio first derivative ultraviolet spectrophotometry. Chem Cent J. 2012;6:2–7.
Chawla A, Sharma A, Sharma A k. Review: a convenient approach for the synthesis of imidazole derivatives using microwaves. Der Pharma Chemica. 2012;4(1):116–40.
Chiririwa H, Muzenda E. Synthesis, characterization of gold (III) complexes and an in vitro evaluation of their cytotoxic properties. Proceedings of the World Congress on Engineering and Computer Science 2014 Vol II (WCECS )2014, 22–24 October, 2014, San Francisco, USA.
De Almeida MV, Saraiva MF, de Souza MVN, da Costa CF, Vicente FRC, Lourenco MCS. Synthesis and antitubercular activity of lipophilic moxifloxacin and gatifloxacin derivatives. Bioorg Med Chem Lett. 2007;17:5661–4.
Drevensˇek P, Kosˇmrlj J, Giester G, Skauge T, Sletten E, Sepc ˇic´ K. X-ray crystallographic, NMR and antimicrobial activity studies ofmagnesium complexes of fluoroquinolones–racemic ofloxacin and itsS-form, levofloxacin. J Inorg Biochem. 2006;100:1755–63.
Efthimiadou EK, Karaliota A, Psomas G. Mononuclear dioxomolybdenium(VI) complexes with the quinolones enrofloxacin and sparfloxacin: synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron. 2008;27:349–56.
Efthimiadou EK, Sanakis Y, Raptopoulou CP, Karaliota A, Katsaros N, Psomas G. Crystal structure, spectroscopic, and biological study of the copper(II) complex with third-generation quinolone antibioticsparfloxacin. Bioorg Med Chem Lett. 2006;14:3864–7.
El-Megharbel SM, Adam AMA, Megahed AS, Refat MS. Synthesis and molecular structure of moxifloxacin drug with metal ions as a model drug against some kinds of bacteria and fungi. Russ J Gen Chem. 2015;85(10):2366–73.
Filimon S-A, Hrib CG, Randoll S, Neda I, Jones PG, Tamm M. Quinine-derived Imidazolidin-2-imine ligands: synthesis, coordination chemistry, and application in catalytic transfer hydrogenation. Z Anorg Allg Chem. 2010;636:691–9.
Gasser G, Ott I, Metzler-Nolte N. Organometallic anticancer compounds. J Med Chem. 2011;13(1):3–25.
Gobec M, Kljun J, Sosič I, Mlinarič-Raščan I, Uršič M, Gobec S, Turel I. Structural characterization and biological evaluation of a clioquinol–ruthenium complex with copper-independent antileukaemic activity. Dalton Trans. 2014;43:9045–51.
Kadyrov AA, Neda I, Kaukorat T, Sonnenburg R, Fischer A, Jones PG, Schmutzler R. New phospholene and phosphepine derivatives irom λ3-phosphorus compounds and hexafluoroacetone or periluorinated α-diketones. Eur J Inorg Chem. 1996;129:725–32.
Khairnar SB, Singh KK. Development and evaluation of moxifloxacin hydrochloride loaded microspheres for controlled release ophthalmic delivery. Int J Pharma Res & Rev. 2016;5(6):23–31.
J. Kljun, A.K. Bytzek, W. Kandioller, C. Bartel, M.A. Jakupec, C.G. Hartinger, B.K. Keppler, I. Turel, Organometallic. 30 (9): (2011) 2506.
Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine. 2010;06(4):570–4.
Kondaiah S, Bhagavanth Reddy G, Rajesh D, Vijayakumr B. Synthesis, characterization and biological activity of moxifloxacin -cu (II) metal complex. IJETSR. 2017;4(12):1138–45.
Kunze C, Neda I, Freitag M, Jones PG, Schmutzler R. Mono-and binuclear rhodium and platinum complexes of 1, 3, 5-Trimethyl-1, 3, 5-triaza-2σ3λ3-phosphorin-4, 6-dionyloxy-substituted Calix [4] arenes. Z Anorg Allg Chem. 2002;628:545.
Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34.
Maftei CV, Fodor E, Jones PG, Franz MH, Kelter G, Fiebig H, Neda I. Synthesis and characterization of novel bioactive 1, 2, 4-oxadiazole natural product analogs bearing the N-phenylmaleimide and N-phenylsuccinimide moieties. Beilstein J Org Chem. 2013;9:2202–15.
Maftei CV, Fodor E, Jones PG, Freytag M, Heiko Franz M, Kelter G, Fiebig H-H, Tamm M, Neda I. N-heterocyclic carbenes (NHC) with 1, 2, 4-oxadiazole-substituents related to natural products: synthesis, structure and potential antitumor activity of some corresponding gold (I) and silver (I) complexes. Eur J Med Chem. 2015;101(28):431–44.
Maftei E, Maftei CV, Heiko Franz M, Kelter G, Fiebig H-H, Tamm M, Neda I. New members of the Cinchona alkaloid family: synthesis, characterization and antitumor evaluation of novel gold (I) complexes. Rev Roum Chim. 2016b;61:251–60.
Maftei E, Maftei CV, Jones PG, Freytag M, Heiko Franz M, Kelter G, Fiebig H-H, Tamm M, Neda I. Trifluoromethylpyridine-substituted N-heterocyclic carbenes (NHC) related to natural products: synthesis, structure and potential antitumor activity of some corresponding gold (I), rhodium (I) and iridium (I) complexes. Helv Chim Acta. 2016a;99:469–48.
Mihorianu M, Heiko Franz M, Jones PG, Kelter MFG, Fiebig H-H, Tamm M, Neda I. N-heterocyclic Carbenes (NHC) derived from Imidazo [1, 5-a] pyridines related to natural products: synthesis, structure and potential biological activity of some corresponding gold (I) and silver (I) complexes. Appl Organometal Chem. 2016;30:581–9.
Muhammad I, Javed I, Shahid I, Nazia I. In vitro antibacterial studies of ciprofloxacin-imines and their complexes with cu(II),Ni(II),co(II), and Zn(II). Turk J Biol. 2007;31:67–72.
Nabd El-Wahed MG, Refat MS, El-Megharbel SM. Spectroscopic, thermal and biological studies of coordination compounds of sulfasalazine drug: Mn(II), hg(II), Cr(III), ZrO(II), VO(II) and Y(III) transition metal complexes. Bull Mater Sci. 2009;32(2):205–14.
Nakamoto K, McCarthy PJ, FuJiwara S, Shimura Y, Fujita J, Hare CR, Saito Y. Spectroscopy and structure of metal chelate compounds. New York, London, Sydney: Wiley; 1968.
Neda I, Farkens M, Fischer A, Jones PG, Schmutzler R. Chemistry of the l,3,5-Triaza-2-phosphinane- 4,6-diones, part V synthesis of phosphoryl(III)(λ4P) and Thiophosphoryl(III)(λ4P) derivatives of 1,3,5-Triaza-2-phosphinane-4,6-diones. Reactions with ketones. Z Naturforsch. 1993b;48b:860–6.
Neda I, Kaukorat T, Schmutzler R, Niemeyer U, Kutscher B, Engel JP u J. Benzodiaza-, benzoxaza- and benzodioxaphosphorinones-formation, reactivity, structure and biological activity. Phosphorus Sulfur Silicon. 2000;162:81–218.
Neda I, Melnicky C, Vollbrecht A, Schmutzler R. An unusual N-alkylation reaction during the oxidative addition of Hexafluoroacetone and Tetrachloro-o-benzoquinone to P-bis-(2-chloroethyl) - substituted *3P compounds. Synthesis –Stuttgart. 1996;4:473–4.
Neda I, Plinta H-J, Schmutzler R. Reactions of 1, 3-Dialkyl-1, 3-diaza-2-chloro-5, 6-benzo-1, 3, 2-phosphorinan-4- ones; preparation of P (III) derivatives. Z Naturforsch. 1993a;48b:333–40.
Ott I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord Chem Rev. 2009;253(11–12):1670–81.
Patel MN, Parmar PA, Gandhi DS. Third generation fluoroquinolones antibacterial drug based mixed-ligand Cu(II) complexes: structure, antibacterial activity, superoxide dismutase activity and DNA-interaction approach. J Enzyme Inhib Med Chem. 2011;26(2):188–97.
Plinta HJ, Neda I, Schmutzler R. 1.3-dimethyl-l, 3-diaza-2-R-5, 6-benzo-2 λ3-phosphorinan-4-ones (R = F, Me2N, 2-methylpiperidino, MeC (: O) NH-) as ligands in transition-metal complexes; synthesis and structure of dichloro-platinum (II) - and tetracarbonyl-metal (0) coordination compounds (metal=Cr, Mo and W).Z. Naturforsch. 1994;49b:100–10.
Power EGM, Phillips I. Correlation between umuC induction and Salmonella mutagenicity assay for quinolone antimicrobial agents. FEMS Microbiol Lett. 1993;112(3):251–4.
Sadeek SA, El-Shwiniy WH, El-Attar MS. Synthesis, characterization and antimicrobial investigation of some moxifloxacin metal complexes. Spectrochim Acta A. 2011;84:99–110.
Rafique S, Idrees M, Nasim A, Akbar H, Athar A. Transition metal complexes as potential therapeutic agents. Biotechnology and Molecular Biology Reviews. 2010;5(2):38–45.
Simon M, Csunderlik C, Cotarca L, Caproiu MT, Neda I, Turoczi MC, Volpicelli R. Synthesis of new active 0-nitrophenyl carbamates. Synth Commun. 2005;35(11):1471–9.
Singh R, Debnath A, Masram DT, Rathore D. Synthesis and biological activities of selected quinolone-metal complexes. Res J Chem Sci. 2013;3(6):83–94.
Soayed AA, Refaat HM, El-Din DAN. Metal complexes of moxifloxacin–imidazole mixed ligands: characterization and biological studies. Inorg Chim Acta. 2013;406:230–40.
Sonnenburg R, Neda I, Fischer A, Jones PG, Schmutzler R. Compounds involving the 5, 6-Benzo-1, 3, 2-diazaphosphorinane-4-one ring system: synthesis of 2-chloro-, 2-N, N-dimethylamino- and 2-Bis-(2-chloroethyl) amino substituted derivatives with three- and four-coordinated phosphorus. Z Naturforsch. 1994;49b:788.
Sultana N, Saeed Arayne M, Akhta M. Synthesis and antimicrobial chattels of 1-cyclopropyl- 7-[(S,S)-2,8-diazabicyclo [4.3.0]Non-8-Yl]-6-fluoro-8- methoxy-1,4-dihydro-4-oxo- 3-quinolinecarboxylic acid metal complexes of biological interest. JSM Chem. 2014;2(1):1007.
Tavares TT, Paschoal D, Motta EVS, Carpanez AG, Lopes MTP, Fontes ES. Platinum(II) and palladium(II) aryl-thiosemicarbazone complexes: synthesis, characterization, molecular modeling, cytotoxicity, and antimicrobial activity. J Coord Chem. 2004;67(6):956–68.
Thomas RE, Papandrea RA. Medical uses of gold compounds: past, present and future. Med J Aust. 1993;158:720.
Trindade MAG, Cunha PAC, de Araújo TA, da Silva GM, Ferreira VS. Interaction study of moxifloxacin with Cu(II) ion using square-wave voltammetry and its application in the determination in tablets. Ecl Quím, São Paulo. 2006;31(1):31–8.
Tulkens PM, Arvis P, Kruesmann F. Moxifloxacin safety an analysis of 14 years of clinical data. Drugs R D. 2012;12(2):71–100.
Turel I. The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev. 2002;32:27–47.
Acknowledgements
The first author would like to thank UGC, New Delhi, India for the financial support from UGC Minor Research Project Grant (Sanction letter no: F.MRP–6210/15(SERO/UGC). The author thanks the Principal and Management of Malla Reddy Engineering College (Autonomous) for providing research facilities.
Funding
University Grants Commission (no: F.MRP–6210/15(SERO/UGC), Delhi, India.
Author information
Authors and Affiliations
Contributions
KS synthesized the moxifloxacin-silver and gold metal complexes. KS, MK, and VB characterized moxifloxacin and their metal complexes using FT-IR and H1NMR spectra. AY and KS characterized the moxifloxacin metal complexes using X-ray diffraction and SEM. SK and VB characterized the moxifloxacin metal complexes using electronic spectra, DSC, elemental analysis, and TGA. KKK conducted antibacterial studies on moxifloxacin and their metal complexes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NA
Consent for publication
NA
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Seku, K., Yamala, A.K., Kancherla, M. et al. Synthesis of moxifloxacin–Au (III) and Ag (I) metal complexes and their biological activities. J Anal Sci Technol 9, 14 (2018). https://doi.org/10.1186/s40543-018-0147-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-018-0147-z