Abstract
Background
A new solid phase extraction (SPE) method has been developed for the separation/preconcentration and determination of lead, cadmium, chromium, nickel, and copper ions using a new sorbent.
Methods
The method is based on the application of multiwalled carbon nanotubes modified with ethylenediamine-N,N-diacetic acid (MWCNTs-EDDA) as a selective sorbent in SPE for the separation and preconcentration of Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III). The determination of these heavy metals was followed by flame atomic absorption spectrometry (FAAS).
Results
All quantitative determinations were performed by FAAS, and surface modification of synthesized adsorbents has been confirmed on the basis of characterization with Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The parameters such as pH, amount of adsorbent, shaking time, eluent type and its concentration, and matrix effect have been investigated and optimized. The maximum adsorption capacity of the adsorbent at optimum conditions was found to be 51.7, 67.6, 49.9, 57.8, and 62.3 mg g-1 for Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II), respectively.
Conclusions
In the presented study, MWCNTs were functionalized by EDDA and it was applied as an adsorbent for the separation and preconcentration of trace level of Pb, Ni, Cd, Cu, and Cr ions. This method is effective, selective, rapid, and simple for the determination of trace amounts of Pb, Ni, Cd, Cu, and Cr ions and can be successfully applied to analyze the trace amounts of mentioned ions in wastewater and herbal samples without evident interference from the complex matrix. Furthermore, the sorption has high capacity, and the precision and accuracy of the method are also satisfactory.
Similar content being viewed by others
Background
Heavy metal pollution on the planet is increasing day by day and has become an environmental problem throughout the world. Heavy metals, such as Cd, Cr, Cu, Ni, As, Pb, and Zn, have high solubility in the aquatic environments and can be absorbed by living organisms, enter the food chain, and accumulate in the human body. Therefore, heavy metals are considered as hazardous materials for ecosystems, animals, plants, and human beings. Heavy metals that can be found in vegetables, ground water, and wastewaters are mostly toxic even at a very low concentration, and the determination of heavy metals is one of the targets of analytical chemists (Babel and Kurniawan 2004; Barałkiewicz et al. 2010; Eskandari et al. 2006; Peker 1999; Turkoglu and Soylak 2005). However, direct determination of heavy metals is often not sensitive enough to determine heavy metal ions in environmental samples because of their low concentrations, which are near or below the detection limit of the instrument, and matrix interferences. Therefore, a preconcentration and/or separation of analytes is frequently necessary to improve the analytical detection limit, increasing the sensitivity, facilitating the calibration, enhancing the accuracy of the results, and simplifying the matrix of the sample (Baghban et al. 2012; Das et al. 2006; Hu et al. 2002a; 2002b; Palanivelu et al. 1992; Prasad et al. 2006; Rajesh and Manikandan 2008; Soylak et al. 2003).
Various separation-preconcentration techniques have been used for the separation and enrichment of trace amounts of heavy metal ions from environmental sample solutions, such as solvent (Lajunen and Kubin 1986) and cloud point (Giokas et al. 2001; Khader and Varghese 2005) extraction, ion-exchange (Möller et al. 1992), and membrane filtration (Karatepe et al. 2002). Despite many benefits, these techniques have significant disadvantages that many researchers prefer solid phase extraction (SPE) because of its notable advantages including higher preconcentration factor, rapid phase separation, short analysis time, cost saving, and low consumption of organic solvents (Absalan and Aghaei Goudi 2004; Liang et al. 2001; Pourreza and Behpour 1999). One of the main factors in SPE procedure is suitable sorbent selection because of its effect on enrichment factor and recovery (Poole 2003). Various adsorbents are used for solid phase extraction of heavy metals, for example, chitosan (Martins et al. 2005), activated carbon (de Peña et al. 1995), resins (Tuzen et al. 2004), and chromosorb 108 (Tuzen et al. 2005). Since carbon nanotubes (CNTs) were discovered by Iijima (Iijima and Ichihashi 1993), because of the large surface area, the excellent chemical and thermal stabilities, and strong adsorption ability, they have attracted great attention as SPE adsorbents for various organic pollutants and metal ions. Due to low dispersion of CNTs into solvents and limited selectivity of CNTs and oxidized CNTs for SPE, a common technique is chemical functionalization which is used relatively often to generate functional groups on the surface of CNTs (Liu et al. 2008).
In this paper, we report a new method using multiwalled carbon nanotubes modified with ethylenediamine-N,N-diacetic acid (MWCNTs-EDDA) as a selective sorbent in SPE for separation and preconcentration of Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III) prior to their determination by flame atomic absorption spectrometry (FAAS). Characterization of synthesized adsorbent has been done using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The effects of variables such as pH, matrix, amount of adsorbents, shaking time, eluent type, and its concentration were studied. Then, the method was applied to the analysis of wastewater and herbal samples with satisfactory results.
Methods
Materials and reagents
Multiwalled carbon nanotubes (MWCNTs) of 95 % purity and a length of 1–10 μm, outer and inner diameter of 5–20 and 2–6 nm, respectively, and 3–15 number of walls were purchased from PlasmaChem GmbH (Germany, http://www.Plasmachem.com). All other reagents such as N,N-dicyclohexylcarbodiimide (DCC), ethyl acetate, bromo-acetic acid, ethylenediamine, sodium hydroxide, concentrated H2SO4 acid, concentrated HNO3 acid, and ethanol were of at least analytical grade and obtained from Merck (Darmstadt, Germany). Standard stock solutions of Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) (1 mg mL−1) were prepared by dissolving analytical or spectral purity grade chemicals Cd(NO3)2.4H2O, CrCl3.6H2O, Cu(NO3)2.3H2O, Ni(NO3)2.6H2O, and Pb(NO3)2 (purchased from Merck) in Milli-Q water and further diluted daily prior to use. Standard glassware used were cleaned with HNO3 and rinsed with Milli-Q water.
Apparatus
All ion determinations were carried out with a flame atomic absorption spectrophotometer (PG Instruments, England) with an air-acetylene flame equipped with PG instrument hallow cathode lamps. A Metrohm pH meter (model 827 pH lab, combined glass-electrode) was employed for the pH adjustment. Fourier transform infrared (FTIR) spectra (4000–400 cm−1) in KBr were recorded by a FTIR Prestige-21, Shimadzu. A Philips TEM (EM 280 model) and a SEM (Tescan Vega II model) were conducted to characterize the raw MWCNTs, oxidized MWCNTs (MWCNTs-COOH), and modified MWCNTs. An ultrasonic bath (S60H Elmasonic, Germany) and a heidolph rotary vacuum evaporator were also used.
Preparation of the solid sorbent
Preparation of oxidized MWCNTs
For elimination of metal ions and other sorbed impurities on MWCNTs (in preparation process), it was purified with HCl (10 %) for 24 h. Then, it was filtered and treated with concentrated HNO3 and refluxed for 3 h with stirring to introduce oxygen groups onto the MWCNT surface (Pyrzynska 2010). The product (MWCNTs-COOH) was filtered through a 0.05-μm pore size PTFE membrane filter, washed with Milli-Q water until the pH was neutral, and dried under vacuum at 80 °C for 8 h.
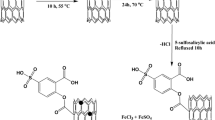
Synthesis of MWCNTs-EDDA
The requirement ligand (EDDA) was synthesized and purified as reported in the literature (McLendon et al. 1975). The oxidized multiwalled carbon nanotubes (2.0 g) were suspended in 150 mL of water, and the synthesized EDDA (0.5 mol) was added, then 2.0 g of DCC was added into it, and the suspension was refluxed for 48 h in 100 °C. The obtained product (MWCNTs-EDDA) was vacuum filtered using a 0.05-μm pore size PTFE membrane filter, washed with excess ethanol, and dried in an oven at 80 °C for 8 h. Figure 1 shows the synthesis process of MWCNTs-EDDA schematically.
General procedure
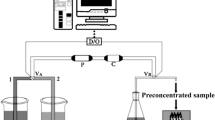
All adsorption experiments in this work were performed in a batch method. Water samples including four industrial wastewater and herbal samples including mushroom, black tea, and lettuce were prepared as reported in the literature (Manzoori et al. 2012). A portion of standard sample, water sample, or digested herbal sample solutions containing the studied ions were added into a 50-mL beaker, and the pH of the mixture was adjusted to the desired value with 0.1 mol L−1 HCl and/or 0.1 mol L−1 NH3.H2O, and the mixture was diluted to 10 mL with deionized water. Then, 15-mg modified MWCNTs were added to it, and the mixture was shaken for 15 min at room temperature to facilitate adsorption of the cations onto the adsorbents. After vacuum filtering, the concentrations of metal ions were determined by FAAS. Finally, 5 mL of 0.5 mol L−1 nitric acid was added as eluent, and the eluted ions were determined by FAAS.
Results and discussion
Characterization of MWCNTs-EDDA
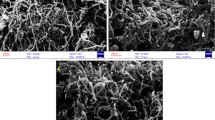
TEM and SEM were conducted to characterize the raw MWCNTs, MWCNTs-COOH, and MWCNTs-EDDA. The representative TEM and SEM images are shown in Figs. 2 and 3, respectively. In contrast, the observed dispersing property of MWCNTs-COOH was better than that of MWCNTs, and the diameter of MWCNTs-EDDA appeared wider than that of MWCNTs and MWCNTs-COOH. These results confirm MWCNTs modified by the EDDA.
Formation of functionalized MWCNTs also was confirmed by IR spectroscopy. The FTIR spectrums of pure, oxidized, and functionalized MWCNTs are compared in Fig. 4. Comparison of the FTIR spectra of MWCNTs with MWCNTs-COOH shows a new bond at 1624 cm−1 in MWCNTs-COOH spectra which can be attributed to the stretching vibrations C=O of the carboxyl group. When MWCNTs-COOH was modified by EDDA, several new peaks appeared in the spectrum. That can be assigned as follows: the peak at 1634 cm−1 is due to C–O stretching vibration. The peak at 1563 cm−1 is caused by C–N stretching vibration and N–H bending vibration.
Effect of pH on adsorption
The pH of the solution is the first parameter that should be optimized, because of two reasons: firstly, the competition of the hydrogen and metal ions for the binding site of sorbent in acidic solutions and secondly, precipitation of metal ions that complex with hydroxide (Walcarius and Delacôte 2005). The effect of pH on the adsorption of lead(II), nickel(II), cadmium(II), copper(II), and chromium(III) ions onto MWCNTs-EDDA was investigated over the range from 2 to 9 using the batch procedure. It could be seen in Fig. 5 that a quantitative extraction (>95 %) occurred in the pH range of 4.5–5.5 for Pb(II), 6.5–7.5 for Ni(II), 7.0–8.0 for Cd(II), 4.5–5.5 for Cu(II), and 3.5–5.0 for Cr(III). Therefore, pHs of 5.0, 7.0, 7.5, 5.0, and 4.0 were selected for further studies of lead, nickel, cadmium, copper, and chromium ions, respectively.
Effect of the sorbent amount
The effect of MWCNTs-EDDA quantity on preconcentration of considered ions was studied in the range of 1–25 mg under the optimum pH and 20-min shaking time. The results show that by increasing the amount of the sorbent, the recovery of metal ions increased due to the increasing surface area and accessible sites of the sorbent and finally reached a constant value at 15 mg of the sorbent. Therefore, 15 mg of MWCNTs-EDDA was selected for future studies.
Effect of shaking time
In solid phase extraction, the percentage extraction of metal ions always depends on the contact time of sample with the sorbent. Herein, a different shaking time (ranged from 1 to 30 min) was studied for the percentage extraction of Pb(II), Ni(II), Cr(III) (10 mL, 10 μg mL−1), Cu(II) (10 mL, 5 μg mL−1), and Cd(II) (10 mL, 2 μg mL−1), with 15 mg of the sorbent. The results show that the adsorption of the mentioned ions at first 10 min was higher than 95 %, but 15 min was used for future studies.
Effect of eluent type and concentration
As shown in Fig. 5, the adsorption of metal ions decreases at low pHs, so a series of acidic solutions including HNO3, HCl, and H2SO4 were used for elution of the retained Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II). The obtained results (Table 1) showed that 5 mL of 0.5 mol L−1 HNO3 solution was suitable for complete elution of retained heavy metals.
Effect of sample volume
Due to low concentration of heavy metals in natural samples, it is necessary to obtain the maximum volume in the SPE. For this purpose, various sample volumes in the range of 10–850 mL containing 100 μg of Pb(II), Ni(II), and Cr(III), 50 μg of Cu(II), and 20 μg of Cd(II) were treated according to the general procedure. It was found that quantitative recoveries (>95 %) were obtained for the sample volumes up to 650 mL for Pb(II), Ni(II), Cu(II), and Cd(II) and 600 mL for Cr(III) ions (Fig. 6). The final solution volume was 5.0 mL, and therefore, the preconcentration factors were 120 for Cr(III) and 130 for Pb(II), Ni(II), Cu(II), and Cd(II) ions.
Effect of sample volume on adsorption of heavy metal ions. Conditions: The conditions are same as Fig. 5 except pH 5.0, 6.0, 9.0, 8.0 and 7.0 for Pb(II), Ni(II), Cd(II), Cu(II) and Cr(III) ions, respectively
Maximum adsorption capacity
The adsorption capacity of MWCNTs-EDDA investigated using the batch method as reported by Maquieira et al. (1994). A portion of sample solutions of various concentrations (10–100 μg mL−1, 10 mL) of Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) were adjusted to the appropriate pH, and all processes of separation and preconcentration were applied on it. The maximum adsorption capacities of MWCNTs-EDDA for Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) were found to be 51.7, 62.6, 49.9, 57.8, and 62.3 mg g−1, respectively. Our results show that untreated and oxidized MWCNTs have much lower adsorption than new synthesized adsorbent.
Adsorption isotherms
Adsorption isotherms were obtained at 25 °C and pH 5.0, 7.0, 7.5, 5.0, and 4.0 for Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III) ions, respectively, and by varying the initial concentration of these ions (Fig. 7a, b). Langmuir and Freundlich isotherms are two commonly used statistical isotherm models. The Langmuir isotherm demonstrates a monolayer coverage of adsorbate with homogeneous sorption energies, while Freundlich isotherm demonstrates a non-uniform distribution of heat of adsorption over the surface with the possibility of multilayer adsorption (Yu et al. 2007). The adsorption data were correlated with the isotherm models of Langmuir (Eq. 1) and Freundlich (Eq. 2)
Adsorption isotherms of a Pb(II), Cr(III), and Ni(II) and b Cd(II) and Cu(II) by MWCNTs-EDDA at initial metal ions concentration from 0.5 to 90 μg mL−1, pH 5.0, 6.0, 9.0, 8.0, and 7.0 for Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III) ions, respectively, and temperature of 25 ± 0.2 °C for 24 h. The data are fitted by Langmuir model and Freundlich model
where C is the equilibrium concentration of metal ions (μg mL−1), q is the equilibrium amount of metal ion adsorbed per unit weight of adsorbents (mg g−1), q max is the maximum adsorption capacity of the adsorbents for metal ions (mg g−1), b is the Langmuir constant and is a measure of the energy of adsorption (L mg−1), k f is the Freundlich constant representing the adsorption capacity, and n is a constant depicting the adsorption intensity. The Langmuir and Freundlich constants were obtained by fitting the adsorption equilibrium data to the isotherm models; they are listed in Table 2. It can be noticed that the R values for the Langmuir model are higher than those of the Freundlich model, thus indicating that the Langmuir model better describes adsorption onto new sorbent.
Effect of interfering ions
Always in real samples, there are many ions and determination of one of them may be influenced by others. Therefore, the effects of interfering ions on the determination of Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) were examined using 10 μg mL−1 of Pb, Ni, and Cr, 5 μg mL−1 of Cu, and 2 μg mL−1 of Cd containing the added interfering ions. The tolerance limit of the interfering ions, defined as the maximum concentration of interfering ion causing a relative error of adsorbed Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II), is less than ±5 %. The results listed in Table 3 show that the suggested method had a good selectivity and can be applied in the determination of studied ions in real samples without any interference of other ions.
Analytical performance and method validation
In order to show the validation of the proposed method, the analytical features of the method such as limit of detection (LOD), linear range of the calibration curve, and precision of ions were examined. Under the optimal experimental conditions, eight standard solutions of Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) were analyzed by the procedure. The LODs were found to be 0.04, 0.15, 0.14, 0.21, and 0.89 μg mL−1 for Cd, Cr, Cu, Ni, and Pb ions, respectively. The linear ranges of calibration curves for Cr(III), Ni(II), and Pb(II) were 0.5–10.0 μg mL−1, 0.5–5.0 μg mL−1 for Cu(II), and 0.2–2.0 μg mL−1 for Cd(II) with correlation coefficients of 0.991, 0.996, 0.997, 0.999, and 0.998, respectively. The regression equations for the lines were A = 0.014CPb + 0.001, A = 0.059CNi + 0.034, A = 0.008CCr + 0.010, A = 0.261CCd + 0.006, and A = 0.085CCu + 0.001, where A is the absorbance and CPb, CNi, CCr, CCd, and CCu are the concentrations of Pb(II), Ni(II), Cr(III), Cd(II), and Cu(II) in μg mL−1, respectively. Intra-day and inter-day relative standard deviations for three individual experiments and 3 days of analysis in water solution were 1.82–3.31 % and 1.63–3.57 %, respectively (Table 4).
Analytical applications
In order to establish the validity of the proposed method, it has been applied for the determination of trace amounts of Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III) in wastewaters from Sarcheshmeh Copper factory and Yazd sewage treatment plant and herbal samples (black tea, mushroom, and lettuce). The results are listed in Tables 5 and 6. According to these data, the added ions can be quantitatively recovered from the samples by the proposed procedure. These results indicate that the method is a selective SPE and determination procedure of trace Pb(II), Ni(II), Cd(II), Cu(II), and Cr(III) in environmental samples.
Conclusions
In the presented study, MWCNTs were functionalized by EDDA and it was applied as an adsorbent for the separation and preconcentration of the trace level of Pb, Ni, Cd, Cu, and Cr ions. This method is effective, selective, rapid, and simple for the determination of the trace amounts of Pb, Ni, Cd, Cu, and Cr ions and can be successfully applied to analyze the trace amounts of the mentioned ions in wastewater and herbal samples without evident interference from the complex matrix. Comparison of this method and some of the previously reported preconcentration methods (Table 7) shows that the LOD and preconcentration factor of this method is comparable or better than achieved by other methods described in the literatures. Furthermore, the sorption has high capacity, and the precision and accuracy of the method are also satisfactory.
References
Absalan G, Aghaei Goudi A (2004) Optimizing the immobilized dithizone on surfactant-coated alumina as new sorbent for determination of silver. Sep Purif Technol 38:209–214
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967
Baghban N, Haji Shabani AM, Dadfarnia S (2012) Solid phase extraction and flame atomic absorption spectrometric determination of trace amounts of cadmium and lead in water and biological samples using modified TiO2 nanoparticles. Int J Environ Anal Chem 93:1367–1380
Barałkiewicz D, Hanć A, Gramowska H (2010) Simultaneous determination of Cd, Cr, Cu, Ni, Pb and Zn in sewage sludge by slurry introduction ICP-OES method. Int J Environ Anal Chem 90:1025–1035
Das AK, Chakraborty R, Cervera ML, de la Guardia M (2006) Analytical techniques for the determination of bismuth in solid environmental samples. Trends Anal Chem 25:599–608
de Peña YP, Gallego M, Valcárcel M (1995) On-line sorbent extraction, preconcentration and determination of lead by atomic absorption spectrometry. Talanta 42:211–218
Eskandari H, Saghseloo AG, Chamjangali MA (2006) First- and second-derivative spectrophotometry for simultaneous determination of copper and cobalt by 1-(2-pyridylazo)-2-naphthol in Tween 80 micellar solutions. Turk J Chem 30:49–63
Ghaedi M, Montazerozohori M, Biyareh MN, Mortazavi K, Soylak M (2012) Chemically bonded multiwalled carbon nanotubes as efficient material for solid phase extraction of some metal ions in food samples. Int J Environ Anal Chem 93:528–542
Giokas DL, Paleologos EK, Tzouwara-Karayann SM, Karayannis MI (2001) Single-sample cloud point determination of iron, cobalt and nickel by flow injection analysis flame atomic absorption spectrometry-application to real samples and certified reference materials. J Anal Atom Spectrom 16:521–526
Hu Q, Guangyu Y, Huang Z, Yin J (2002a) Spectrophotometric determination of silver with 2-(2-quinolylazo)-5-diethylaminoaniline. Talanta 58:467–473
Hu Q, Yang G, Yin J, Yao Y (2002b) Determination of trace lead, cadmium and mercury by on-line column enrichment followed by RP-HPLC as metal-tetra-(4-bromophenyl)-porphyrin chelates. Talanta 57:751–756
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature 363:603–605
Karatepe AU, Soylak M, Elci L (2002) Separation/preconcentration of Cu(II), Fe(III), Pb(II), Co(II), and Cr(III) in aqueous samples on cellulose nitrate membrane filter and their determination by atomic absorption spectrometry. Anal Lett 35:1561–1574
Khader AMA, Varghese A (2005) Simultaneous determination of titanium and molybdenum in alloy steels using derivative spectrophotometry in neutral micellar medium. Indian J Chem Technol 12:707–707
Kiran K, Suresh Kumar K, Suvardhan K, Janardhanam K, Chiranjeevi P (2007) Retracted: Preconcentration and solid phase extraction method for the determination of Co, Cu, Ni, Zn and Cd in environmental and biological samples using activated carbon by FAAS. J Hazard Mater 147:15–20
Lajunen LHJ, Kubin A (1986) Determination of trace amounts of molybdenum in plant tissue by solvent extraction-atomic-absorption and direct-current plasma emission spectrometry. Talanta 33:265–270
Liang P, QinY HB, Peng T, Jiang Z (2001) Nanometer-size titanium dioxide microcolumn on-line preconcentration of trace metals and their determination by inductively coupled plasma atomic emission spectrometry in water. Anal Chim Acta 440:207–213
Liu Y, Li Y, Yan XP (2008) Preparation, characterization, and application of l-cysteine functionalized multiwalled carbon nanotubes as a selective sorbent for separation and preconcentration of heavy metals. Adv Funct Mater 18:1536–1543
Liu Y, Li Y, Wu ZQ, Yan XP (2009) Fabrication and characterization of hexahistidine-tagged protein functionalized multi-walled carbon nanotubes for selective solid-phase extraction of Cu2+ and Ni2+. Talanta 79:1464–1471
Manzoori J, Amjadi M, Darvishnejad M (2012) Separation and preconcentration of trace quantities of copper ion using modified alumina nanoparticles, and its determination by flame atomic absorption spectrometry. Microchim Acta 176:437–443
Maquieira A, Elmahadi HAM, Puchades R (1994) Immobilized cyanobacteria for online trace metal enrichment by flow injection atomic absorption spectrometry. Anal Chem 66:3632–3638
Martins AO, da Silva EL, Laranjeira MCM, de Fávere VT (2005) Application of chitosan functionalized with 8-hydroxyquinoline: determination of lead by flow injection flame atomic absorption spectrometry. Microchim Acta 150:27–33
McLendon G, Motekaitis RJ, Martell AE (1975) Cobalt complexes of ethylenediamine-n, n’-diacetic acid and ethylenediamine-n, n-diacetic acid. Two-nitrogen oxygen carriers. Inorg Chem 14:1993–1996
Möller P, Dulski P, Luck J (1992) Determination of rare earth elements in seawater by inductively coupled plasma-mass spectrometry. Spectrochim Acta B 47:1379–1387
Ozcan SG, Satiroglu N, Soylak M (2010) Column solid phase extraction of iron(III), copper(II), manganese(II) and lead(II) ions food and water samples on multi-walled carbon nanotubes. Food Chem Toxicol 48:2401–2406
Palanivelu K, Balasubramanian N, Ramakrishna TV (1992) A chemical enhancement method for the spectrophotometric determination of trace amounts of arsenic. Talanta 39:555–561
Peker I (1999) The solubilities of Zn, Sb and Se found in anode slime. Asian J Chem 11:979–986
Poole CF (2003) New trends in solid-phase extraction. Trends Anal Chem 22:362–373
Pourreza N, Behpour M (1999) Column preconcentration of aluminum using eriochrome cyanine R and methyltrioctylammonium chloride adsorbent supported on naphthalene with subsequent spectrophotometric determination. Microchem J 63:250–256
Prasad K, Gopikrishna P, Kala R, Rao TP, Naidu GRK (2006) Solid phase extraction vis-à-vis coprecipitation preconcentration of cadmium and lead from soils onto 5,7-dibromoquinoline-8-ol embedded benzophenone and determination by FAAS. Talanta 69:938–945
Pyrzynska K (2010) Carbon nanostructures for separation, preconcentration and speciation of metal ions. Trends Anal Chem 29:718–727
Rajesh N, Manikandan S (2008) Spectrophotometric determination of lead after preconcentration of its diphenylthiocarbazone complex on a Aamberlite XAD-1180 column. Spectrochim Acta A 70:754–757
Soylak M, Karatepe AU, ElÇİ L, Doǧan M (2003) Column preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using amberlite XAD-1180. Turk J Chem 27:235–242
Turkoglu O, Soylak M (2005) Spectrophotometric determination of copper in natural waters and pharmaceutical samples with chloro(phenyl) glyoxime. J Chin Chem Soc 52:575–579
Tuzen M, Soylak M (2007) Multiwalled carbon nanotubes for speciation of chromium in environmental samples. J Hazard Mater 147:219–225
Tuzen M, Soylak M, ElÇİ L, Doǧan M (2004) Column solid phase extraction of copper, iron, and zinc ions at trace levels in environmental samples on Amberlite XAD‐7 for their flame atomic absorption spectrometric determinations. Anal Lett 37:1185–1201
Tuzen M, Soylak M, Elci L (2005) Multi-element pre-concentration of heavy metal ions by solid phase extraction on chromosor B 108. Anal Chim Acta 548:101–108
Tuzen M, Saygi KO, Soylak M (2008) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632–639
Vellaichamy S, Palanivelu K (2011) Preconcentration and separation of copper, nickel and zinc in aqueous samples by flame atomic absorption spectrometry after column solid-phase extraction onto MCWNTs impregnated with D2EHPA-TOPO mixture. J Hazard Mater 185:1131–1139
Walcarius A, Delacôte C (2005) Mercury(ii) binding to thiol-functionalized mesoporous silicas: critical effect of ph and sorbent properties on capacity and selectivity. Anal Chim Acta 547:3–13
Yang B, Gong Q, Zhao L, Sun H, Ren N, Qin J, Xu J, Yang H (2011) Preconcentration and determination of lead and cadmium in water samples with a MnO2 coated carbon nanotubes by using ETAAS. Desalination 278:65–69
Yu J, Tong M, Sun X, Li B (2007) Cystine-modified biomass for Cd(II) and Pb(II) biosorption. J Hazard Mater 143:277–284
Acknowledgements
The Iran National Science Foundation (INSF) is gratefully acknowledged for financial support of this work (Project No. 90002933).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MAK, AM and SZM conceived of the original idea and design of the work. JY performed the collection of water and herbal samples, preparation of reagents and solutions and execution of experiments. MAK and AHM performed the data interpretation and manuscript writing. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Karimi, M.A., Mohammadi, S.Z., Hatefi-Mehrjardi, A. et al. A new sorbent based on MWCNTs modification for separation/preconcentration of trace amounts of Cd(II), Cr(III), Cu(II), Ni(II), and Pb(II) and their determination by flame atomic absorption spectrometry. J Anal Sci Technol 6, 25 (2015). https://doi.org/10.1186/s40543-015-0065-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-015-0065-2