Abstract
Amyotrophic Lateral Sclerosis (ALS) is the most frequent motor neuron disease in adults. Classical ALS is characterized by the death of upper and lower motor neurons leading to progressive paralysis. Approximately 10 % of ALS patients have familial form of the disease. Numerous different gene mutations have been found in familial cases of ALS, such as mutations in superoxide dismutase 1 (SOD1), TAR DNA-binding protein 43 (TDP-43), fused in sarcoma (FUS), C9ORF72, ubiquilin-2 (UBQLN2), optineurin (OPTN) and others. Multiple animal models were generated to mimic the disease and to test future treatments. However, no animal model fully replicates the spectrum of phenotypes in the human disease and it is difficult to assess how a therapeutic effect in disease models can predict efficacy in humans. Importantly, the genetic and phenotypic heterogeneity of ALS leads to a variety of responses to similar treatment regimens. From this has emerged the concept of personalized medicine (PM), which is a medical scheme that combines study of genetic, environmental and clinical diagnostic testing, including biomarkers, to individualized patient care. In this perspective, we used subgroups of specific ALS-linked gene mutations to go through existing animal models and to provide a comprehensive profile of the differences and similarities between animal models of disease and human disease. Finally, we reviewed application of biomarkers and gene therapies relevant in personalized medicine approach. For instance, this includes viral delivering of antisense oligonucleotide and small interfering RNA in SOD1, TDP-43 and C9orf72 mice models. Promising gene therapies raised possibilities for treating differently the major mutations in familial ALS cases.
Similar content being viewed by others
Introduction
Amyotrophic Lateral Sclerosis (ALS) is the most common motor neuron disorder in adults. It is characterized by progressive death of upper and lower motor neurons. This degeneration leads to paralysis and to patient death within 2 to 5 years after disease onset. In the last ten years, a wide variety of gene mutations have been discovered for the familial form of the disease (fALS), leading to an impressive genetic heterogeneity. Expanded hexanucleotide repeats in C9orf72 account for nearly 35 % of familial cases, mutations in superoxide dismutase 1 (SOD1) for 20 %, mutations in TAR DNA-binding protein (TARDBP) encoding TDP-43 and fused in sarcoma (FUS) for 4 % each. Other genes like p62 (SQSTM1), Ubiliquin-2 (UBQLN2), TANK-binding kinase 1 (TBK1) and Optineurin (OPTN) account for less than 1 % each [1] (Fig. 1). Genetic heterogeneity and other unknown causes of the sporadic form of ALS (sALS) lead to a phenotypic variability which increases treatment complexity of the disease. Numerous pathological cellular mechanisms are identified in ALS and have been recently reviewed [2]. This includes oxidative stress, mitochondrial defect, axonal transport impairment, protein aggregation, excitotoxicity, endoplasmic reticulum stress, abnormal RNA processing and neuroinflammation with a role of non-neuronal cells (Fig. 1). These mechanisms will not be further discussed in this review.
Timeline of gene discovery and pathogenic mechanisms in ALS. Schematic representation of years of discovery of most important genes implicated in ALS. Mutation in the superoxide dismutase 1 (SOD1) represent approximately 15 % of familial ALS cases (fALS), mutation in C9orf72 represent 35-40 % and both TAR-DNA-binding protein (TARDBP) and Fused in sarcoma (FUS) for 4 % each. Other genes represent less than 1 % each. Protein aggregation and gliosis are pathological hallmark of ALS and have been discovered before the beginning of the illustrated timeline. ER endoplasmic reticulum; NEFH neurofilament heavy; ALS2 alsin; DCTN1 dynactin; PRPH peripherin; SETX senataxin; VAPB vesicle-associated membrane protein-associated protein B; CHMP2B Charged multivesicular body protein 2B; ANG angiogenin; FIG4 phosphoinositide 5-phosphatase; OPTN optineurin; ATXN2 ataxin 2; DAO D-amino acid oxidase; SPG11 spastic paraplegia 11; VCP valosin containing protein; SIGMAR1 sigma non-opioid intracellular receptor 1; TAF15 TATA-box binding protein associated factor 15; UBQLN2 ubiquilin-2; SQSTM1 sequestosome 1; PFN1 profilin-1; HNRNPA1 heterogeneous nuclear ribonucleoprotein A1; ERBB4 erb-2 receptor tyrosine kinase 4; MATR3 matrin 3; TUBA4A tubulin alpha-4a; TBK1 TANK-binding kinase 1
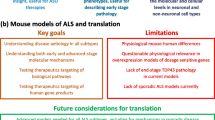
Development of transgenic animal models is the primary stage for understanding pathophysiology and for testing future effective therapies [3]. The broad variety of genetic mutations identified in ALS and ALS with frontotemporal dementia (ALS-FTD) leads to extensive literature about ALS animal models. Some of these models may raise concerns about their validity for human disease because of their incomplete or differing phenotypes and the lack of treatment reproducibility in humans. In this review, we summarize ALS animal models in mice, rats, fruit flies, worms, zebrafish, dogs and pigs. We compare each model to the clinical presentation of ALS-FTD and discuss the relevance of these models. We also discuss therapies which can be applied to specific gene mutation in a perspective of personalized medicine. For the moment, Riluzole is the only drug approved for ALS treatment. Numerous other drugs established to be efficient in mice have failed in clinical trials [4]. Interestingly, many of these drugs were effective in a small proportion of patients but were unable to exhibit a favorable effect on overall trial. These curious results are probably caused by the high variability in clinical phenotypes and biological pathways found in ALS and underscore the need for evaluating a personalized medicine approach to treatment.
Review
Clinical manifestation and epidemiology of amyotrophic lateral sclerosis
Classical ALS
ALS affects both upper (UMN) and lower motor neurons (LMN). It has a wide phenotypic variability determined primarily by three main features: site of onset, rate of progression and relative number of UMN and LMN deficits [5]. Classical spinal onset generally starts with an asymmetric weakness in a limb and the patient will consult a physician because of an unexplained foot drop with or without falls [6]. Weakness will progressively spread to other contiguous limb regions and finally reach respiratory muscles after a few months [5]. Clinical examinations could reveal lower motor neurons signs which include weakness, muscle atrophy, fasciculations and reduced reflexes, whereas upper motor neurons signs include hypertonia, hyperreflexia, Babinski’s sign and Hoffman’s sign [7] (Fig. 2). The onset of symptoms is gradual. It is uncommon for patients to complain about sensory deficit or paresthesia. However, electrophysiological and pathological studies confirmed that response to stimuli can be altered and a loss of large caliber axon was observed [8, 9]. Furthermore, a combination of spinal diffusion tensor imaging (DTI) and electrophysiological recordings demonstrated a subclinical sensory deficit in 85 % of patients with ALS [10].
Clinical findings in Amyotrophic lateral sclerosis (ALS). Signs and symptoms are divided by affected motor neuron. Both upper motor neurons (UMN) and lower motor neurons (LMN) have to be affected for the diagnosis of ALS. Different combination of LMN and UMN signs can be observed. Limb onset is found in around 65 % of patients but most patients will develop signs in both bulbar region and limbs within the course of disease. Up to 50 % of ALS patients may have symptoms of Fronto-Temporal dementia
Bulbar ALS
Approximately 20 to 30 % of ALS starts with bulbar onset. Lower motor neuron sign in bulbar onset include facial weakness, low palatal elevation, dysarthria, tongue fasciculations and atrophy. Upper motor neuron signs include slow speech, slow tongue movement, palmomental reflex and jaw jerk reflex [11] (Fig. 2). Change in phonation and uncontrolled crying and laughing, known as pseudobulbar affect, can be present [12]. A differential diagnosis has to be made with progressive bulbar palsy, which is characterized by dysphagia and dysarthria with predominant lower motor involvement. ALS prognosis is generally worse with bulbar onset because of the early respiratory dysfunction.
Fronto-temporal dementia
Cognitive impairment in ALS patients frequently occurs in the evolution of disease. These symptoms range from small cognitive impairment (50 %) to official diagnosis of fronto-temporal dementia (FTD). FTD, in up to 15 % of ALS patients, generally presents with personality change, behavioural abnormalities, language dysfunctions and memory impairment [13]. FTD is the second most common form of dementia after Alzheimer’s disease. It is characterized by progressive degeneration of frontal and anterior temporal lobes. Both ALS and FTD share common pathological mechanisms such as TDP-43 cytosolic aggregation [14]. Furthermore, recent findings suggest that a single genetic mutation can cause both diseases, together or separately. C9orf72 and TDP-43 are strongly linked to both disorders whereas SOD1 is rarely mutated in FTD cases [15].
Epidemiology
The incidence of amyotrophic lateral sclerosis is 1 to 3 per 100,000 person/years and is relatively similar between countries [16–18]. Usually men have a higher risk (1.2 to 1.5) of developing ALS during their lifetime [19]. The lifetime risk for men is approximately 1:350 and 1:400 for women [20]. Age at onset differs between familial and sporadic forms of ALS. Patients with affected relatives normally develop the disease in their forties or early fifties whereas sporadic cases on average develop it in the late fifties [21–23]. There is also a peak of incidence in the sporadic form between 70-79 years.
Several risk factors for ALS are proposed but none of them have clearly established pathophysiology. Men who served in the military are at higher risk (RR = 1.53; 95 % CI: 1,12 to 2.09) of developing ALS without any regard to war and service [24]. Incidence is also higher among football players (40 fold) and soccer players (6.5 fold) [25, 26]. Some studies have suggested a possible association between physical activity and development of ALS but more work has to be done to confirm this hypothesis [27]. Additionally, head traumas, which persist as major health concern among football and other collision sports players, are being studied as risk factors to ALS. Neuropathology studies described TDP-43 proteinopathy in brains and spinal cords of athletes with ALS-FTD [28]. Finally, lower body mass index and smoking are linked to a higher risk of ALS [29, 30].
Neuropathology
Charcot first described the neuropathological features of ALS which consist of muscle atrophy, loss of anterior horn cells and sclerosis of the spinal cord lateral columns. Degeneration affects most of the motor neuron system. Nevertheless, the nuclei controlling eye movement (oculomotor, trochlear and abducens) and fecal and urinary continence (Onufrowicz’s) are generally intact [31]. Motor neurons show histopathological feature such as cytosolic inclusions. Mutation specific cellular structures will be detailed in the following sections. No macroscopic changes are observed in the brain of most ALS patients [32]. However, atrophy of the frontal and temporal cortex including reduced white matter volume can be observed with magnetic resonance imaging (MRI) in ALS-FTD [33, 34]. Combined techniques of voxel-based morphometry (VBM) and MRI demonstrated white matter deficits along corticospinal tract, corpus callosum, cerebellum and in frontal and occipital subcortical regions [35].
Astrocytes and microglia activation, defined as gliosis, is a pathological hallmark of ALS. Analysis from sporadic and familial ALS revealed microglial activation, reactive astrocytes and T cell infiltration in the in spinal cord [36, 37]. Positron emission tomography has brought more evidence of activated microglia in brain of ALS patients. Activated microglia was found in the motor cortex, pons, dorsolateral prefrontal cortex and thalamus. The authors also described a positive correlation between microglia activation in motor cortex and amount of upper motor neuron signs [38].
Diagnostic criteria
The El Escorial criteria (EEC) have been employed since 1990 for the diagnosis of ALS and have been revised in 2000 [39]. Diagnosis is based upon UMN signs, LMN signs, the identified gene mutation, electrophysiological and neuroimaging studies, but no definitive diagnostic test are suitable for ALS. For definite ALS, UMN and LMN clinical signs in three different regions are needed. However, EEC is excessively restrictive as some patients are dying without meeting criteria for definite ALS [40]. For example, a patient without an identified gene mutation and with only LMN signs will be classified as clinically suspected ALS or probable ALS with laboratory supports, if present. More recently, the Awaji-Shima criteria was introduced to improve the sensitivity of ALS diagnosis and increases potential entry into clinical trials [41]. Diagnosis of definite ALS required clinical or electrophysiological evidence of UMN and LMN features in one bulbar region and two spinal regions or in three spinal regions. Consequently, ALS can be detected with electrophysiological evidence in clinically unaffected regions of an early stage patient. The most frequent electrophysiological evidences in ALS are fasciculation, fibrillation potentials, positive sharp waves and polyphasic units. A meta-analysis revealed that Awaji-Shima criteria have a sensitivity of 81.1 % as compared to 62.2 % for EEC and Awaji criteria are also better for diagnostic of bulbar onset [42].
Mutation in familial cases of ALS
Superoxide dismutase 1 (SOD1)
Specific disease characteristics in humans
Mutations in the SOD1 gene have been the first described cause of familial ALS [43]. Since 1993, more than 150 missense mutations have been described in SOD1, all updated on the ALSoD website (http://alsod.iop.kcl.ac.uk/). Unfortunately, individual mutations are poorly correlated with clinical presentation. Furthermore, the clinical phenotype is often variable between members of the same family. Almost all clinical manifestations of ALS can be observed with SOD1 mutations [23, 44–47]. However, some authors have stated that the SOD1 A4V mutation leads to a rapid death after only one year of symptoms [22] and that people with D90A mutations have slow disease progression [45]. Some specific characteristics for SOD1 mutations are listed in Table 2. Age at onset can vary from 6 to 94 years old with a mean of 40 ± 9.9 to 58.9 ± 12.6 according to studies. Progression of the disease can also vary from 8 months to 18.7 ± 11.4 years. Most of the time, familial SOD1 ALS starts with an asymmetric weakness in a limb with predominantly lower motor neurons signs. Patients usually suffer from weakness, atrophy, fasciculation and reflexes can be either increased or decreased. Babinski’s sign is often absent. Cognitive symptoms are usually not present in familial SOD1 cases. However, FTD has been described with four SOD1 mutations, G41S, L144F, I113T and G141X [48–51]. Non-motor symptoms have been described mainly with the D90A mutation, such as urinary symptoms, painful muscle spasm, cerebellar ataxia and sensory symptoms [45, 52].
Neuropathological findings described in patients with SOD1 mutations include several classes of cytoplasmic inclusions. Lewy-body like hyaline inclusions (LBHI) are the most described inclusions in SOD1 variants. They have been found in patients with A4V [53], G37R [54], H46R [55], H48Q [56], I113T [57] and L126S [58] mutations. LBHI, in hematoxylin and eosin (H&E) staining, exhibits dense cores with paler peripheral halos. These inclusions are usually composed of granule-coated fibrils, mutated and wild-type SOD1 and ubiquitin [59]. Other bodies, like intracytoplasmic SOD1 inclusions, skein-like inclusion or neurofibrillary tangle, were found in SOD1 familial cases (Table 2). Hyaline inclusions are also present in astrocytes [60].
Mouse models
The first animal model carrying a mutation in human SOD1 was created in 1994 [61]. SOD1G93A was expressed under the control of the human SOD1 promoter. These mice reproduced most of the clinical and neuropathological findings of ALS. They developed motor deficits in Rotarod and Hangwire tests at 80-90 days and died at 130 days after loss of muscle innervation and motor neuron degeneration. Reports have demonstrated degeneration of the neuromuscular junction before the onset of symptoms, around 40 to 50 days [62, 63]. Gliosis was found before onset and increased in intensity over time [64]. Also, one report has showed an increased proportion of activated microglia from 80 days of age [65] and more recently, positron emission tomography (PET) imaging in the SOD1G93A mouse showed increased inflammation by 110 ± 33 % in the whole brain [66].
It was originally proposed that the pathological effect of SOD1 in ALS was caused by a loss of dismutase function. However, Sod1 knock-out (KO) mice did not develop ALS up to six months of age [67]. Further studies suggest that Sod1 KO mice develop a significant distal motor axonopathy without any motor neuron loss [68]. Indeed, the role of SOD1WT in ALS pathology has to be clarified. Some studies have demonstrated that SOD1WT was not implicated in neurodegeneration of ALS. Mice expressing wild-type human SOD1 developed vacuolization of mitochondria and axonal degeneration in the spinocerebellar tract but without motor neuron loss before 2 years of age [69]. However, co-overexpression of SOD1WT in fALS-mutant mice succeeded to increase the ALS phenotype and to convert unaffected mice to ALS [69, 70]. More recently, expression of SOD1WT at same level as seen in SOD1G93A mouse caused an ALS-like phenotype with similar neuropathological findings [71].
Numerous SOD1 mouse models have been created and they have variable phenotypes, age of disease onset and survival (Table 3). These heterogeneous phenotypes seems to be dependent on specific mutations, expression levels of mutant SOD1, gender and genetic background [72]. Generally, females experienced delayed onset and prolonged survival as compared to males and such differences can also be observed in humans [73]. Most SOD1 mutant mice represent human SOD1 pathology quite well. Mice develop fatal paralysis with motor neuron deficit, gliosis and intracytoplasmic ubiquitinated SOD1 inclusions (Fig. 3). However, poor correlation can be made between age of onset and progression in mouse and human. For example, the A4V mutation, the most frequent mutation in humans which causes a rapid disease, is not pathogenic in mouse before 85 weeks [74]. Moreover, the SOD1 G93A mutation, which leads to an early onset and fast progression in mouse, has a slow rate of progression in human (Tables 1 and 2). Interestingly, the D90A mutation in mice and humans shows strong similarities. Homozygous SOD1D90A mice exhibit a slow disease progression and bladder disturbance, which are also found in humans with the same homozygous mutation [45, 75]. Cognitive symptoms have been described in some mouse models. Mice exhibiting the G37R mutation have learning deficits in passive avoidance from 8 months of age and pre-symptomatic SOD1G93A mice exhibit learning delay and long-term memory deficits [76, 77]. Non-motor features such as sensory deficits are described in G37R and D83G [78].
Neuropathological findings in human sALS cases and animal models of ALS. Microscopic pictures of neuropathological findings in ALS models. Our previously published TDP-43 and SOD1 mouse models were exploited for illustration of TDP-43 and SOD1 aggregates with permission. a Immunofluorescence microscopy of a hSOD1G93A mouse spinal cord. The B8H10 antibody was utilized for the specific signal of misfolded SOD1. Pictures were taken at 10x and b 40x magnification for better visualization of aggregates. c-e Double immunofluorescence microscopy of 10 months-old a hTDP-43G348C mouse spinal cord using hTDP-43 monoclonal antibody and d ubiquitin antibody [138]. Ubiquinated TDP-43 cytoplasmic aggregates can be observed and are typical neuropathological findings in human ALS. f-i Immunofluorescence of 10 months-old hTDP-43G348C and non-transgenic mice spinal cord. Iba1 antibody f-g and GFAP antibody h-i showed increased microgliosis and astrogliosis in a 10 months-old hTDP-43G348C mouse. j-l Immunohistochemistry of two human sporadic ALS cases using TDP-43 antibody to illustrated typical neuronal cytoplasmic TDP-43 inclusions in lumbar spinal cord (j), medulla (k) and motor cortex (l). Scale bar = 250 μm (a); 50 μm (b, f-i); 25 μm (c-e); 100 μm (j-l)
Mouse models have also been created to understand the role of SOD1 in specific central nervous system (CNS) cells. Neuron-specific high expression of SOD1G93A under Thy1.2 promoter led to motor neuron degeneration and paralysis [79] but lower expression of SOD1G37R under the NF-L promoter did not cause motor neuron deficit [80]. This inconstancy can probably be explained by level of hSOD1 expressed and the specific mutation used. A group also took advantage of the Cre recombinase to decrease expression of the SOD1G37R mutant in astrocytes and they observed delayed microglial activation and slowed late disease progression [81]. Mice expressing the G86R mutant restricted to astrocytes did not develop motor deficit or microglial activation but showed increased Glial fibrillary acidic protein (GFAP) reactivity [82]. These results suggest that glial cells contribute to disease progression but are not sufficient to trigger motor neuron disease.
Other models
H46R and G93A are the only two human SOD1 mutations introduced in rat (Table 2). These rats developed ALS features similar to SOD1 mouse models. They develop UMN and LMN degeneration and similar neuropathological findings. SOD1G93A rats also have motor neuron (MN) loss in trigeminal, facial and hypoglossal nuclei which correspond to features of bulbar ALS [83]. Rats are of particular interest for ALS research because of their size which facilitate intra-thecal or intra cerebro-ventricular injection for preclinical trials.
Canines can be affected by degenerative myelopathy (DM), a progressive neurodegenerative disorder with robust similarities to human ALS, making dogs the only mammals with naturally occurring non-human ALS [84]. DM is particularly similar to the UMN-dominant onset form of ALS. The onset of clinical signs generally occurred at 8 years of age and duration of disease rarely exceed 3 years because of elective euthanasia. T18S and E40K mutations in SOD1 were reported in dogs with DM [85, 86]. Similarities between both diseases include axonal degeneration, astrocytosis, fibrillation and positive sharp waves at electromyography evaluation, muscle atrophy, sensory impairment and SOD1 inclusions [84]. However, MN loss is not clearly established in DM as some authors reported MN loss in dogs with DM [87] whereas others did not [88].
Zebrafish have a simplified vertebrate nervous system which offers accessible motor neurons for in vivo study of ALS. They are particularly promising for fast screening of new potential therapeutic approaches as response to treatment can be rapidly observed. However, even if the zebrafish CNS shares similarities with human CNS, structural differences are important to consider when analyzing these models. Comparative neuroanatomy was recently reviewed in [89]. For example, the absence of corticospinal and rubrospinal tracts in zebrafish make it a poor model for UMN disorders. Zebrafish injected with mutant SOD1 mRNA display abnormal branching and reduced motor neuron axonal length, but did not become paralysed. In contrast to mice, zebrafish injected with the SOD1 A4V mRNA exhibit the most prominent phenotype [90]. Transgenic G93A and G93R zebrafish exhibit MN and neuromuscular junction (NMJ) loss with deficits in swimming [91, 92].
Drosophila melanogaster is a useful tool for genetics research given its low maintenance cost as compared to rodents and other mammals. It also has shorter lifespan which allows for fast initial studies of diseases and treatment. However, the mammalian nervous system is much more complex and additional studies have to be realized in mammals before translating results directly from Drosophila to human. Human SOD1WT, SOD1A4V and SOD1G85R were expressed in Drosophila [93]. All of these transgenic flies exhibit climbing inability at two to four weeks of age, suggesting a motor deficit. A decrease in synaptic transmission was observed without MN loss. The authors also observed a stress response in glial cell through increased immunostaining of Hsp70.
Caenorhabditis elegans is a nematode which can be cultured in agar plates and reach a length of 1 mm [94]. C. elegans possess the advantage of being transparent thus allowing easy detection of fluorescent proteins. Their short lifespan permits the rapid screening of drugs and the extensive study of molecular pathways. However, the simplicity of their nervous system, as in Drosophila and zebrafish, do not allow the direct translation of discoveries and treatment to humans. Human SOD1WT, SOD1G85R, SOD1G93A and SOD1 with mutations in four cysteine residues (C4S) were introduced into C. elegans. Motor neuron deficits were measured by the number of “thrashing” movements by the worm, which are periodical changes of direction when placed in physiological buffer. Overexpression of hSOD1WT and SOD1G85R caused motor deficit leading to paralysis within 10 to 20 days [95].
In last few years, pigs have been used for modeling disorders such as Parkinson disease [96], Alzheimer’s disease [97] and Huntington disease [98]. Their role in modeling disease is based on their anatomical, genetic and physiological resemblance to human. CNS anatomy is particularly closer to human than the rodents. Minipigs expressing hSOD1G93A under the CMV promoter were produced [99]. From 3 months of age, the pigs develop hind limb defects resulting in running difficulty and this incapacity became more severe with age. The pigs also exhibit muscle atrophy and accumulation of SOD1 in motor neurons but did not die of the disease up to two years of age.
Biomarkers
Interest in biomarkers in ALS research has growth in the last decades. Unfortunately, no biomarkers are validated yet for the diagnosis of ALS. The discovery of biomarkers will help early diagnosis and potentiate therapeutic intervention in a context of personalized medicine. The optimized biomarker will be quantifiable, will be standardized, will have low variability and will be introducible within all centers. Volatile organic compounds (VOC), which are tools for biomarker investigation, have been analyzed in SOD1G93A mice [100]. Mice, in early stage of the disease, exhibit a pattern of 12 different oxidative stress-related blood VOCs as compared to non-transgenic aged-matched mice. MicroRNAs (miRNAs) are also of particular interest in biomarker development as they are frequently altered in disorders, can be dosed in biological fluids and the pattern of variation in miRNA level is relatively specific. miRNA-206 has been identified in the blood and muscles of SOD1G93A symptomatic mice [101]. miRNA-206 was also increased in blood of ALS patients, but without any discrimination of familial or sporadic form of disease. However, a recent report suggests that the miRNA pattern of expression in serum is mutation-independent. Consequently, stratified subgroups could increase the significance of miRNA as biomarkers. Indeed, patients with FUS and C9orf72 mutations have a similar serum miRNA profile compared to SOD1 patients, but profiles of miRNA was less similar among SOD1 patients [102]. Actually, among all ALS patients, a serum miRNA signature was observed decade before disease onset as compared to healthy controls. Imaging techniques are also potential biomarkers for ALS. SOD1G93A mice exhibit a tissue vacuolization in T2 MRI studies. These alterations occurred before MN loss and matched histopathological change in post-mortem analysis [103]. Another group also observed vacuolization and gliosis in T2 MRI as soon as 60 days in SOD1G93A [104].
Personalized medicine
Personalized medicine (PM) is a medical scheme that combines study of genetic, environmental and clinical diagnostic testing, including biomarkers, to individualize patient care. Patients are divided into stratified subgroups to improve their response to treatment [105]. This concept has emerged from the need to understand the variety of response to similar treatment in common illnesses. The application of personalized medicine in ALS first requires genetic screening among ALS patients in clinical trials and in overall neurology clinics and eventually, utilization of biomarkers for personalized diagnosis and treatment. Ideally, subgroups of specific mutations should be applied in clinical trials because of the high phenotypic variability of these mutations (Table 2). Unfortunately, recruitment difficulty is limiting this approach. As better drugs become available, it will be important to take into account the genetic profile status of the patient to determine if individuals with certain mutations would respond better to particular treatments. This has been successful in other diseases such as the use of Vemurafenib in melanoma patients specifically with a BRAF V600E mutation [106].
SOD1 animal models exhibits most clinical features found in humans with SOD1 mutations and this is particularly accurate for rodents and canine. This accuracy suggests that results in SOD1 rodents can potentially be applied to human with SOD1 mutations in a context of PM. However, the review of the literature suggest that SOD1 models are less accurate for modeling general ALS since they exhibit important disparities with sALS and other forms of fALS. SOD1 transgenic mice do not recapitulate the C-terminal and phosphorylated TDP-43 cytosolic inclusions observed in almost all familial and sporadic ALS cases [107, 108]. Likewise, Lewy body-like inclusions are a typical neuropathological finding of SOD1 patients and animal models but are not observed in sALS and SOD1-unrelated fALS [109].
Gene therapy is a promising avenue for personalized medicine in ALS. Recombinant adeno-associated viral (AAV)-mediated gene delivery is the most developed method for gene therapy. These vectors can target specific cells when directly injected in the CNS or by crossing the blood-brain barrier when injected systemically. AAVs expressing the insulin growth factor 1 (IGF-1) and glial cell line-delivered neurotrophic factor (GDNF) injected in muscle succeed to prolong life-span and delay disease in the SOD1G93A mouse by retrograde action on MN [110]. Intra-thecal injection of AAV expressing a single-chain antibody against misfolded SOD1 also delayed disease onset and extended life-span up to 28 % in SOD1G93A mice [111]. It is clearly established that SOD1 mutants develop a toxic gain of function. Consequently, silencing the SOD1 gene by delivery of a lentiviral vector that expresses small interfering RNAs (siRNA) was tested in SOD1G93A. The authors described a delay in disease onset by more than 100 % and a life-span extends by 80 % [112]. A delay in onset and extension of life-span were also observed in SOD1G93A rats injected with AAV9-SOD1-shRNA in the motor cortex [113] and in SOD1G93A mice treated with intravenous injection of AAV9-SOD1-shRNA [114]. With the same idea, antisense oligonucleotide targeting the SOD1 gene infused in the lateral ventricle of SOD1G93A rats extends survival by 10 days and extends disease duration by 37 % [115]. Actually, a recent phase 1 clinical trial of intrathecal injection of antisense oligonucleotide was conducted in SOD1 familial ALS cases and no serious adverse effects were identified [116]. A phase 2 clinical trial is currently conducted but no results are yet available.
TAR-DNA-binding protein (TDP-43)
Specific disease characteristics in humans
TAR-DNA-binding protein 43 (TDP-43) is a multi-functional DNA/RNA binding protein normally found in the nucleus. It is known to play a role in RNA processing and transport and splicing regulation [117]. TDP-43 was found to be a major component of pathologic cytosolic inclusions in ALS and FTD [118, 119]. TDP-43 was subsequently found to be mutated in familial and sporadic ALS patients [120–123]. While TDP-43 mutations are rare (4 % of fALS), it is notably established that the TDP-43 protein is found in inclusions of most ALS cases. Over 40 mutations are currently identified but G298S, A315T, M337V, G348C, and A382T are the most frequent among patients [124].
It is currently clinically impossible to clearly discriminate TDP-43 patients from other sALS and fALS patients. It is also difficult based on the literature to establish clear phenotype-genotype correlation within each variant of TDP-43 mutation. Clinical characteristics of frequent mutations are outlined in Table 3. However, one publication succeeds to point out some specific clinical characteristic in TDP-43 mutated patients [124]. Age at onset can vary from 20 to 77 years old with a mean age at onset of 54 years old [125]. M337V and G348C have the earliest disease onset. Onset within TDP-43 patients is significantly earlier than sALS patients but no difference can be observed when compared to SOD1 patients, which also have earlier disease onset [124]. The upper limb is the predominant site at onset comprising 60.7 % of patients [124]. Bulbar onset seems more frequent in Asian patients (55 %) as compared to Caucasians (24.7 %) and in patients with a M337V mutation. Small cohort of patients with TDP-43 mutations was used and makes these results difficult to generalize.
Fronto-temporal dementia is relatively rare within TARDBP ALS cases. A382T is the most described mutation in FTD-TDP-43 familial cases [126–129]. One group identified a Sardinian cohort of ALS with an A382T mutation with frequent dementia (30.8 %) [130]. They also identified that the A382T mutation is present in 21.7 % of their FTD cohort [126]. However, a similar frequency of TDP-43 mutations was not reported in other populations. The authors explained that this frequency is probably caused by the isolated population of Sardinia with its associated founder effects. Clinical features of FTD cases with an A382T mutation mainly include irritability, aggressiveness, poor flexibility, fixed ideas, change in eating behaviors, trouble in social behaviors and emotional flatness [126]. Brain imaging studies also suggested a pattern of defect found in A382T related FTD cases. MRIs showed unilateral or bilateral frontotemporal atrophy and frontotemporal hypoperfusion, observed in SPECT brain perfusion. In combination, neuroimaging findings showed prevalent involvement of temporal regions, which is rarely reported within other familial FTD cases [126].
Neuropathological findings in TDP-43 fALS patients tend to represent the majority of sALS and fALS cases. Regardless of the numerous publications on TDP-43, only a few reports are available with neuropathological findings. Indeed, most of our knowledge about TDP-43 neuropathological features comes from mouse models. In patients, neurons and glial cells are positive for TDP-43 cytosolic inclusions [131]. Often, these inclusions are also composed of ubiquitin and P62. Bunina bodies are described in most mutations except in A315T.
Mouse models
The discovery of TDP-43 as a major component of ALS inclusions had led to the generation of many mouse models trying to recreate this pathology (Table 4). In ALS patients, levels of the TDP-43 protein are elevated 1.5-2.5 fold in pathologic neurons [132, 133]. The first strategy for modeling ALS with TDP-43 transgenic mice was to overexpress hTDP-43WT to similar level found in humans. Many available hTDP-43WT models are based on high protein expression by the mouse prion (mPrp) or Thy1.2 promoters. The phenotype observed in these mice seems to be dependent on TDP-43 protein level and did not reproduce the age-related degeneration of ALS. Hemizygous mice expressing 3–4 fold levels of hTDP-43WT did not recapitulate features of ALS [134]. These mice did not develop motor dysfunction and had only mild gliosis and diffuse ubiquitin staining. However, homozygous mouse expressing 2.5 fold increases in TDP-43 levels had rapid onset and low survival [135]. Mice died within approximately 2 months with associated motor deficits. Nevertheless, these mice did not recapitulate classical ALS neuropathological findings. Phospho-TDP-43 inclusions were rare and no motor neuron loss was observed. Mice expressing high level of hTDP-43WT under Thy1.2 promoter (3.8–5.1 folds) also developed rapid progression of disease and had 30 % UMN and 25 % LMN loss with rare phospho-TDP-43 aggregates. Some reports have proposed that the fast disease progression without important loss of motor neurons could be caused by gastrointestinal complications before appearance of full neurodegeneration [136, 137].
Our group took advantage of genomics fragments including the human endogenous TDP-43 promoter to moderately express hTDP-43WT, hTDP-43A315T and hTDP-43G348C in mouse [138]. These mice develop age-related progressive motor deficit in the rotarod test from 36–42 weeks of age and cognitive impairment suggestive of FTD. We also observed ubiquitin/TDP-43 nuclear and cytosolic inclusions in 10 month old hTDP-43A315T and hTDP-43G348C mice (Fig. 3). Nonetheless, these mice did not become paralysed and died at standard age. Interestingly, mPrp-hTDP-43A315T mice develop disease and develop paralysis within few months, but no TDP-43 neuronal cytosolic inclusions (NCI) were observed [139]. As shown in Table 4, no clear correlation can be made between mutation and mouse phenotypes. Correlation can be observed though between the levels of protein expression and phenotypes. Low protein expression caused age-related motor dysfunction and TDP-43 cytosolic accumulation, but the mice did not get paralysed [138, 140]. On the other hand, high protein expression cause early onset and fast disease progression without clear motor neuron loss and TDP-43 NCI. More recently, double transgenic mice expressing hTDP-43WT and hTDP-43Q331K developed rapid progressive limb paralysis starting from 3 weeks of age and leading to death by 8–10 weeks [141]. TDP-43WT and TDP-43Q331K single transgenic mice do not develop motor dysfunction up to 24 months of age. These double transgenic mice also exhibited NCI positive for p62, ubiquitin and TDP-43, reproducing features of inclusions found in human cases. They also exhibited MN loss in the anterior horn of the spinal cord with association of muscular atrophy and NMJ loss. These mice recapitulated most ALS features in exclusion of age-related degeneration.
Other models
Few TDP-43 rat models have been generated. Mild expression of hTDP-43 with its endogenous promoter did not cause motor deficits in rats [142]. However, rats expressing the M337V mutation under endogenous TDP-43 promoter developed fast motor deficit following by paralysis and death within 10 to 29 days. To have a delayed phenotype and to successfully establish transgenic lines, the authors used a Tet-off system and activated TDP-43 expression 4 days after birth. These rats develop symptoms from 3 weeks of age and die at 5 weeks. TDP-43 was observed within the cytoplasm but no clear inclusions were detected. Interestingly, the rats exhibited degeneration of the ventral root, dorsal root and corticospinal tract. Specific neuronal expression of hTDP-43M33V under the neurofilament heavy (NFH) promoter with a Tet-off system expressed at 60 days also caused rapid disease onset [143]. Rats became paralysed within 3–4 weeks after doxycycline induction of TDP-43 expression in neurons. Again, no TDP-43 neuronal cytoplasmic inclusions were detected but ubiquitin inclusions were detected in rats when hTDP-43M337V was expressed in motor neurons.
Multiple Drosophila melanogaster models for TDP-43 have been generated to understand the cellular role of TDP-43. Many of them do not represent ALS because of their tissue expression and poor motor phenotype. They have been extensively described in a recent review on Drosophila melanogaster and ALS [144]. Interesting models of Drosophila were created with the D42-Gal4 system [145–147]. These flies expressed the TDP-43 transgene exclusively in motor neurons. hTDP-43WT overexpression in Drosophila leads to fast paralysis and death in the larval stage [145]. Features of ALS were not clearly demonstrated since these flies died rapidly and no NCI or motor neuron loss was established. Later, hTDP-43WT and hTDP-43A315T overexpressing flies were found to have motor deficit in a climbing assay [146]. Interestingly, cytosolic aggregates of TDP-43 were observed in another model of hTDP-43WT Drosophila [148]. These flies also developed dose-dependent loss of NMJ with adult-onset motor disorder. They exhibit progressive loss of motor ability leading to complete paralysis at 30 days and control flies kept 20 % of motor aptitude at same age. Recently, thoracic motor neuron loss was observed in flies expressing temperature-dependant dTDP-43WT [147].
Transgenic Caenorhabditis elegans models of TDP-43 are also based on protein overexpression. Two groups have expressed different forms of TDP-43 under control of the snb-1 promoter [149, 150]. They observed a motility defect and degeneration phenotype which was worse with mutant TDP-43. Some worms became paralysed and died within 3 weeks. Phosphorylated TDP-43 was detected but no TDP-43 NCI were noted. Moreover, injection of mutant TDP-43 mRNA in Zebrafish embryos caused motor neuron axonopathy [151, 152]. No phenotype was observed upon overexpression of WT-TDP-43 alone.
Biomarkers
Few TDP-43 biomarkers are available in literature and none of them are clearly validated for disease screening in human. ELISA quantification demonstrated an elevated TDP-43 protein level in plasma of ALS cases as compare to healthy subjects [153]. No differences were established between sporadic and familial cases. The same finding was observed in the cerebrospinal fluid (CSF) of ALS patients [154]. Interestingly, TDP-43 CSF level was higher within the first 10 months of disease onset. TDP-43 CSF level was significantly higher than the level measured in other neurologic disorder such as Parkinson’s disease, multiple sclerosis, Guillain-Barré syndrome and progressive supranuclear palsy [155]. A limit dose of 27.9 ng/ml of TDP-43 protein in CSF had a sensitivity of 59.3 % and a specificity of 96.0 %. These results suggest that TDP-43 CSF level could be an interesting tool for ALS diagnosis. Recently, tissue-engineered skins derived from ALS patients demonstrate that skin could possibly be used as biomarker as well. TDP-43 aggregation was observed in sALS-derived skin and in not yet symptomatic patients carrying GGGGCC DNA repeats [156].
Personalized medicine
According to our review of the literature, most TDP-43 models exhibit only a part of all important ALS features. We used five major criteria to point out the mice models which are more representative of human disease. Mice should have TDP-43 cytosolic inclusions, motor neuron loss, age-related onset of disease, paralysis which cause shorter lifespan, and gliosis. In most models, the rapid onset does not correlate with development of ALS in older patients and thus is difficult to use as a neurodegenerative model. Generally, mice with late onset do not get paralysed and miss one the major aspect of ALS pathogenesis. We have highlighted models which filled most of the criteria (Bold in Table 4). We consider that these models should be preferred for drug testing.
Promising approaches for the treatment of TDP-43 cases have been generated in the last few years. We have highlight therapies which can be applied to specific treatment of TDP-43 cases in a context of personalized medicine. Induced pluripotent stem cells (iPSCs) derived from TDP-43M337V patients have been used for gene therapy testing [157]. A reduction of 30 % of cytosolic TDP-43 and 45 % of nuclear TDP-43 level was observed in M337V-iPSCs transfected with a siRNA specifically targeting M337V allele. This approach has to be tested in animal models but seems to be a potential treatment of TDP-43 fALS cases. IPSCs will certainly help to develop therapies in the next years. Specific mutations in patients could be targeted by siRNAs. It is well accepted that TDP-43 mutations disturb cellular RNA metabolism. One of the surveillance mechanisms in cells is composed of upframeshift protein 1 (UPF1) which destroys mRNAs with premature codon. AAV-expression of UPF1 in TDP-43 mice ameliorates motor phenotype and blocked paralysis of forelimbs [158]. These results suggest that gene therapy could also be used for overexpression of surveillance mechanisms instead of directly targeting the TDP-43 protein.
C9ORF72
Specific disease characteristics in humans
A hexanucleotide (GGGGCC) repeat in a non-coding region of C9orf72 was first described in 2011 in ALS, FTD and ALS-FTD familial cases [159, 160]. C9orf72 frequency varies geographically but seems to be present in up to 35–45 % of fALS, making it the most common genetic cause. The normal number of GGGGCC repeats is variable within healthy persons; approximately 90 % have fewer than 10 repeats. Conversely, the number of repeats can reach hundreds to thousands in ALS patients. The role of these repeats in ALS-FTD physiopathology remains unclear. However, recent semi-automated quantification of expansion number exposed a link between the number of G4C2 repeats and clinical characteristics [161]. FTD patients have shorter disease duration with a higher number of repeats, but no correlation was observed in ALS patients.
C9orf72-related ALS cases have several distinct characteristics. Nevertheless, like other genes, a wide range of clinical phenotype can be observed in patients carrying C9orf72 expansions. Age at onset of C9orf72 (C9ALS) fALS patients vary between studies [162–164]. There is good evidence that bulbar onset is more frequent in C9ALS patients than non-C9orf72 patients [162, 164, 165]. Also, there is certainly a higher prevalence of FTD in C9ALS cases. Co-morbid dementia was observed in 50 % of C9orf72 patients and only in 12 % of ALS cases without the expansion [164]. C9orf72-related FTD is mainly characterized by a higher frequency of psychotic symptoms and irrational behaviour as compared to other causes of FTD [166]. Interestingly, hypokinesia/bradykinesia, rigidity and sometimes, tremor, suggestive of parkinsonism, have been described in C9ALS patients [167]. Members of this family were either touched by ALS, FTD and/or progressive supranuclear palsy (PSP). Generally, parkinsonism symptoms seen in these patients are unresponsive to levodopa treatment. C9orf72 expansion was rare in the diagnosis of Parkinson’s Disease (PD) [168, 169].
Particular features can be seen in imaging studies of C9orf72-related ALS/FTD and are dependent of the presence or absence of FTD symptoms. PET imaging identified hypometabolism in anterior and posterior cingulate, insula, caudate and thalamus, and hypermetabolism in the midbrain, bilateral occipital cortex, globus pallidus and left inferior temporal cortex as compared to sALS patients [170]. Voxel-based morphometry revealed atrophy in right frontal gyrus, in left anterior cingulate gyrus and in right precentral gyrus of C9ALS patients [164].
TDP-43 pathology is largely observed in the motor system of C9ALS patients [171]. There is also a large amount of P62 positive and TDP-43 negative inclusions in the pyramidal cells, hippocampus and cerebellum of C9ALS patients [172]. A recent review of the literature has help to understand the neuropathological findings associated with G4C2 repeat cases [173]. It is important to note that while the G4C2 repeat occurs within intron 1 of the C9orf72 gene, the repeat expansion has been shown to nonetheless be translated in both sense and antisense orientations to generate proteins with dipeptide repeats that then are found in intracellular inclusions. The prevalence of TDP-43, p62 and dipeptide protein repeat (DPR) inclusions was assessed specifically in the CNS. In the spinal cord, TDP-43 NCI was observed in 84.1 %, P62 NCI in 70.6 % and DPR inclusions in 49.8 % of patients. P62 and DPR were the major component of NCI in the cerebellum (93.8 and 90.9 % respectively) and TDP-43 was found in only 3.9 % of cerebellums. P62 and DPR were more prevalent than TDP-43 in the hippocampus but TDP-43 was more prevalent in the substantia nigra.
Mouse models
The first mice carrying GGGGCC repeats expansions were recently generated [174] (Table 5). These mice carried 80 G4C2 repeats controlled by TRE promoter. After doxycycline induction, the mice developed ubiquitin-positive inclusion but no DPR were observed. The authors suggest that this model can further be used for the study of toxicity induced by RNA. However, no behavioral analysis was conducted on these mice. Knockout of C9orf72 in mice did not result in any motor neuron degeneration or reduced survival, which suggests that loss-of-function is not sufficient to cause ALS [175]. More recently, an AAV vector expressing either 2 or 66 repeats of G4C2 were injected into CNS of postnatal day 0 mice [176]. RNA nuclear foci were observed in mice carrying 66 repeats but not in control mice carrying only 2 repeats. Also, rare pTDP-43 aggregates were observed in the nucleus and cytosol of cortex and hippocampus regions. These aggregates were not positive for poly(GA) but 75 % of cells containing TDP-43 NCI were positive for poly(GA) inclusions. (G4C2)66 mice exhibit anxiety behavior in an open field test and motor impairment in the second day of rotarod testing at 6 months of age as compared to control mice.
Recently, two different groups generated mice carrying a bacterial artificial chromosome (BAC) with 100 to 1000 GGGGCC repeats [177, 178]. These mice did not exhibit any behavioral or motor phenotypes. However, they developed sense/antisense intranuclear RNA foci and DPR in both neurons and glial cells. Both groups suggest that this number of repeat is sufficient to cause RNA accumulation but not to cause cellular dysfunction leading to motor disease in mice. ALS patients with only a few hundred copies have been reported. Thereby, this discrepancy between mice and human has to be clarified. Very recently, two other groups generated transgenic mice carrying BAC with approximately 450 and 500 GGGGCC repeats [179, 180]. Jiang et al. have generated a mouse model that exhibits cognitive deficits from 12 months of age in working memory test and anxiety evaluation [179]. The mice also exhibit age-related RNA foci and DPR cytosolic accumulation. Liu et al. have generated and well characterized a mice model which developed many features of C9ALS [180]. These mice developed muscle denervation, MN loss, anxiety-like behavior, degeneration in hippocampus, paralysis and decreased survival. RNA accumulation was age-related and the authors also found TDP-43 inclusions in degenerating brain.
Other models
Whether nuclear RNA foci or DPR, or both, are toxic for cells remain an important question and this is currently under investigation by many groups. As previously described, Drosophila melanogaster is a superior tool for understanding pathological mechanisms than mimicking disease phenotype. Two recent publications suggested that DPR are more toxic to cells than nuclear RNA foci by generation of transgenic drosophila [181, 182]. In both studies, drosophila containing DPR had decreased survival but no phenotype was observed in flies with expression of RNA-only repeats. However, motor impairment was not described. 30 GGGGCC repeats were expressed under the motor neuron specific promoter Ok371-GAL4 in D. melanogaster [183]. With Drosophila activity monitoring (DAM) system, the authors observed a significant motor impairment at 28 days after eclosion. Neuronal death was observed in cell culture and in the eye but was not confirmed in motor neurons of flies. Another drosophila model was generated with 58 repeats of G4C2 in motor neurons using the Ok371-GAL4 promoter [184]. These flies developed impaired locomotor activity in larvae, a decrease in bouton number of NMJ and a low muscle area.
Deletion of the C9orf72 orthologue, alfa-1(ok3062), in C. elegans, caused a motor phenotype [185]. The worms developed an age-related motility defect reaching paralysis in 60 % of worms at 12 days of adulthood. The authors also crossed alfa-1(ok3062) with their FUSS57Δ and TDP-43A315T model and observed that the motor phenotype was worse in the double transgenic alfa-1(ok3062);TDP-43A315T C. elegans as compared to simple TDP-43 mutant worms. However, no synergistic effect was observed in alfa-1(ok3062); FUSS57Δ worms. The loss of function theory was also studied in zebrafish. Knockdown of the zebrafish C9orf72 orthologue was established using antisense morpholino oligonucleotides [186]. Fish developed motor neuron axon abnormalities, swimming impairment in reaction to touch and also reduced spontaneous swimming 48 h post-fertilization. No information was given on survival of zebrafish.
Biomarkers
Multiple biomarkers have been studied as diagnostic tool or disease progression marker in C9orf72 pathology despite the relatively recent discovery of its implication in ALS. One of the most characterized cellular findings within C9orf72 patients is the presence of nuclear RNA foci. RNA foci are found in many cell types such as skin biopsy-derived fibroblasts, leukocytes and lymphoblasts and can possibly be utilized as a disease progression marker [187]. Also, a recent report suggests that the level of 5’ methylation of the G4C2 repeat can inversely correlate with disease duration in 34 C9orf72 patients [188]. This epigenetic modification can be found in blood, spinal cord and frontal cortex. Another potential biomarker tool is the level of poly-GP proteins in a patient’s CSF [189]. When compared to healthy subjects and ALS patients without C9orf72 repeat expansion, C9-ALS patients have significantly increased levels of poly-GP peptide in their CSF. Further studies have to be performed to clarify peptide levels in each disease progression stage.
Personalized medicine
It is premature to establish which C9orf72 model is better for mimicking human disease. Animal models of C9ALS repeat expansion have to be optimized and extensively characterized. However, based on clinical presentation, some specific characteristics are central features of C9 cases and should be found in animals. This includes RNA foci, DPR and TDP-43 positive aggregates, age-related disease and reduced survival. Cognitive symptoms are also commonly found in C9ALS patients. Most generated mice develop cellular features of C9ALS patients such as RNA foci and DPR [176–178]. Recently, two groups have generated promising models which exhibits most of C9ALS features and might be exploited for drug testing [179, 180].
Although many therapeutic targets are conceivable, only a few have been currently tested. First, targeting RNA with antisense oligonucleotide (ASO) therapeutics should be a potential avenue because of RNA toxicity in C9ALS models. ASO were tested in fibroblasts and iPSCs derived from C9ALS ALS patients and succeed to decrease RNA foci without reducing overall RNA levels [190, 191]. ASO managed to reduce glutamate toxicity and increased survival of iPSCs by 30 % [190]. Tolerability of C9orf72 reduction in mouse was then tested and no pathological effects were observed on motor ability, strength and anxiety up to 17 weeks after ASO treatment by intracerebroventricular injection [191]. More recently, a single-dose ASO injection which targets repeat-containing RNAs caused a reduction in RNA foci and DPR in mice expressing BAC C9ORF72 RNA with 450 repeats [179]. Reduction in anxiety and cognitive function were also observed at 9 months and a positive effect on behavioral was sustained 6 months after injection. These results suggest a promising treatment possibility for C9orf72 patients.
Fused in sarcoma (FUS)
Specific disease characteristics in humans
Genetic screenings have identified mutations in the gene encoding fused in sarcoma (FUS) [192–194]. Similarly to TDP-43, the main function of FUS is linked to RNA metabolism. More than 50 mutations have been identified and mutations in FUS represent around 4 % of fALS and 1 % of sALS. Unfortunately, there are only a few clinical descriptions of FUS pathology and phenotypic correlation is difficult to establish. Many reports suggest that juvenile onset is more frequent in FUS cases. A German study reports a series of families with age at onset ranging from 21 to 76 years old and with many cases before the age of 40 [195]. They also suggest that truncating mutations have a more severe disease course than missense mutations. LMN signs appear to be the dominant features in FUS cases. Cognitive symptoms are particularly rare with only few cases reported in the literature and bulbar/spinal onset are both frequent with a mutation in FUS [196].
Neuropathological findings in FUS-related disease include basophilic inclusions which are round or oval often similar in size to the nucleus. These inclusions are mainly found in the anterior horn of the spinal cord and sometimes in the motor cortex [197]. These inclusions are commonly found in juvenile ALS with mutations in FUS. NCI are also present in pathological analysis and are similar to those observed in TDP-43 pathology. They are often positive for p62 but negative for TDP-43 [32].
Mouse models
FUS KO mice were reported to investigate the effect of FUS deletion. Mice either died in the first 24 h of life or had important deficits including sterility and chromosomal instability [198]. Another group suggested that knock-out FUS mice were developing a neuropsychiatric disorder that did not correspond to ALS [199]. Overexpression of hFUSWT under the mouse prion promoter leads to an aggressive phenotype in homozygous mice [200] (Table 6). These mice had approximately 1.9 times the level of FUS expression. They develop motor impairment leading to paralysis from 8 weeks of age and were euthanized when 10–13 weeks old. They exhibit increased cytoplasmic FUS without ubiquitinated-FUS inclusions, MN loss in the anterior horn of the lumbar spinal cord, impaired NMJ and gliosis. Mice expressing FUSWT or FUSR521G driven by the ubiquitously expressed enhancer-chicken β-actin hybrid (CAG) promoter were also generated [201]. The mice exhibited early mortality before post-natal day 30 without any FUS inclusions or degeneration of lateral columns and ventral horns. However, gliosis and motor impairment in rotarod tests were observed.
Other models
An encouraging hFUSR521C rat model was generated using a tetracycline-inducible system [202]. After doxycycline withdrawal, the mutant FUS protein was expressed in offspring. The rats developed many features of ALS pathology. Degenerating axons were observed in the spinal cord ventral roots, dorsal roots, corticospinal tracts and frontal cortex of hFUSR521C rats but not in hFUSWT rats. Muscle atrophy was observed and electromyography shown fibrillation potential which is suggestive of muscular atrophy. FUS-negative ubiquitin aggregates were detected in transgenic hFUSR521C rats. Glial activation was also detected in the brain and spinal cord. Nevertheless, the rats developed fast paralysis leading to death within 30 to 70 days of age.
Several Drosophila melanogaster models were generated for investigating the pathological role of FUS. First, the Gal4-UAS system was used for expression of WT, R524S or P525L mutations in motor neurons [203]. Morphological changes were observed in the cell body and at NMJ leading to functional deficits of MN in transgenic flies. The authors observed significant locomotive impairment in larval movement in flies expressing either of three FUS variants. Conditional expression of FUS variants with the elav-GeneSwitch system demonstrated a shorter lifespan of transgenic flies [204]. The authors observed a faster rate of death and presence of cytosolic accumulation of FUS protein in flies expressing mutated FUS as compared to flies expressing WT-FUS. Interestingly, the level of non-aggregated but insoluble FUS protein positively correlated with the level of neurodegeneration in transgenic Drosophila [205].
Caenorhabditis elegans was also used for modeling FUS pathology and interesting results were obtained from this model. A group expressed different forms of FUS including WT, R514G, R521G, R522G, P525L and two truncated FUS [206]. Only R522G, P525L and the truncated forms of FUS caused aggregates and only these transgenic worms exhibited a motor phenotype. Motor impairment started at 3 days of age and C. elegans became paralysed at 8 days of age. Survival was reduced by 12.8 days in worms expressing R522G, P525L or truncated FUS as compared to non-transgenic C. elegans. These results were partially confirmed by expression of either WT or S57Δ FUS in GABAergic neurons [207]. Motor phenotypes were observed only in FUSS57Δ worms which expressed FUS cytosolic inclusions.
Zebrafish harboring mutations in FUS gene were also generated. FUSR521H but not FUSR521C or FUSS57Δ fishes exhibited motor impairment when compared to FUSWT [208]. High-speed video analyses of touch-evoked locomotor activity revealed shorter swim duration, swim distance and swim velocities in fish expressing FUSR521H as compared to wild type fish [209]. NMJ synaptic transmission was also reduced.
Personalized medicine
Our review of the literature has pointed out rat and mouse models with many phenotypic and neuropathological features of ALS [200, 202]. However, they do not possess age-related characteristic of ALS. We consider that these models could be adequate models for drug testing relative to FUS pathology. There is sparse literature which can be applied to a personalized medicine approach in FUS cases. One group has tried to identify biomarkers in skin derived fibroblasts from sporadic ALS patients. Unfortunately, the study failed to identify differences in FUS pattern of expression in skin between healthy control and ALS cases [210]. Like previously described, mi-RNA are potential biomarkers in ALS. FUS is known to be implicated in metabolism of a subset of micro-RNAs such as miR-9, miR-125b and miR-132 [211]. Their value as biomarkers remains to be tested. Methylation is an important process in nuclear-cytoplasmic shuttling of FUS. One group tried to reduce FUS cytosolic accumulation by reducing their methylation with shRNA targeting the FUS methylation enzyme [212]. Reduction of FUS cytoplasmic inclusions was noted.
Other genes
Multiple other genes were identified in familial ALS cases including Ubiquilin-2 and Optineurin (Fig. 1). These mutations remain rare and little data are available for the clinical presentation of these forms. Thus, more work has to be done before a personalized medicine approach in these patients can be comprehensively reviewed.
Ubiquilin-2 (UBQLN2)
Ubiquilin-2 plays a central role in the ubiquitin proteasome system (UPS). Mutations in UBQLN2 have been linked to ALS and FTD [213] though screening in different populations has revealed that mutations in UBQLN2 are generally rare within ALS cases [214, 215]. However, the UBQLN2 protein is found in cytosolic inclusions of both familial and sporadic ALS and appears to have an important role in pathological processes such as aggregate formation and proteasome impairment [213, 216]. No clear genotype-phenotype correlation can be established in patients. FTD appears to be frequent in ALS caused by mutation in UBQLN2. Both males and females can be affected despite the X-linked transmission [217]. Some studies have suggested early age at onset in familial UBQLN2 ALS and site at onset was described in lower limbs, upper limbs and bulbar regions [218]. A specific pattern of UBQLN2 staining has been observed in C9orf72 patients and the authors proposed that this staining could be used as biomarker for identification of C9orf72 cases [219].
Mice carrying a hUBQLN2P497H mutation under control of the UBQLN2 endogenous promoter were recently generated [220]. These mice develop UBQLN2/ubiquitin/p62 positive inclusions in the brain, dendritic spinopathy and cognitive deficits at 11–13 months of age which suggest features of FTD. However, the mice do not develop motor impairment or motor neuron loss. Conversely, AAV expression of three different UBQLN2 variants by intracerebroventricular injection in mice caused a phenotype at 3–4 months of age [221]. Transgenic UBQLN2P497H or knock-out rats were also created [222]. While cognitive and neuronal loss was observed in UBQLN2P497H rats, no phenotype developed in knock-out rats. Astrocytes and microglial activation was observed but no information was given about motor function.
Optineurin (OPTN)
The Optineurin (OPTN) protein is mainly implicated in the autophagy processes. A mutation in the OPTN gene was first linked to ALS in a consanguineous Japanese family [223]. A neuropathological study suggested that OPTN was present in skein-like inclusions and round hyaline inclusions in the spinal cord of sALS patients [224]. However, another study mentioned that OPTN inclusions are rare and restricted to a minority of sALS cases [225]. Clinical characteristics were investigated in patients carrying Q398X and E478G mutations [226, 227]. These patients exhibit slowly progressive motor dysfunction, unusual finger malformations and personality changes. Observed NCI were positive for TDP-43, p62 and ubiquitin but negative for OPTN. OPTN inclusions were described in a patient with both C9orf72 hexanucleotide repeats and an OPTN mutation [228]. Both spinal and bulbar onsets are described but no strong conclusions can be made about duration of the disease and age at onset. Generation of a transgenic mouse with mutation in OPTN gene failed to demonstrate any motor phenotype [229]. Similarly, loss of OPTN in Zebrafish results in cell death but no motor phenotypes were noted [230].
Conclusion
ALS is a fatal disease with large genetic and phenotypic heterogeneity which leads to a variety of responses to similar treatment regimens. There is currently a strong need for treatment discovery to help patient care. For that purpose, animal models which exhibit human disease characteristics have to be optimized. Most of current identified genetic mutations have corresponding animal models. We hope that this review will increase the awareness on qualities and weakness of these models and will eventually help researchers to take advantage of the best model available.
Personalized medicine approaches allow physicians to group together patients with similar characteristics. This could be performed with the use of biomarkers and over time with the same mutated gene. We have reviewed specific treatments which could be applied to sub-groups of patients with ALS. We consider that gene therapy has great potential for personalized medicine approaches, either by antisense oligonucleotide, small interference RNA or any other method such as antibodies targeting pathological proteins (Fig. 4). These techniques have already been tested and appear to be effective in SOD1, TDP-43, C9ORF72 and FUS animal models [111–115, 157, 179, 212]. We are optimistic that the use of gene therapy will growth in clinical trials in the next few years. Promising technologies for delivering genes have been suggested and revealed many procedures for safely targeting central nervous system. Lentiviral or AAV injections or peripherally injected exosomes which specifically target neurons are within these auspicious avenues.
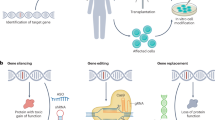
Gene therapy mechanism of action. Schematic representation of possible gene therapy approaches in ALS treatment. All of these approaches can be effective by intra-thecal, intracerebroventricular or peripheral injection of AAV or lentivirus targeting motor neurons or glial cells. a Antisense olinucleotide (ASO) are short synthetic oligonucleotides (15-25 nucleotides) which bind to targeted mRNA. ASO reduces the expression of a specific protein by two main mechanisms. ASO induces the mRNA degradation by endogenous RNase H or blocks the mRNA translation. This is a potential therapeutic avenue in ALS by reducing the protein level of TDP-43, SOD1 of FUS protein level or by targeting of C9orf72 RNA foci. b SiRNAs are double-stranded RNAs which operated through RNA interference pathway. After strand unwinding, one siRNA strand binds argonaute proteins as part of the RNA-induced silencing complex (RISC) and is recruited to a target mRNA which is then cleaved. c Antibodies are another potential therapeutics avenue in ALS [111]. Antibodies can target misfolded proteins and reduce the amount of toxic aggregates. It is suggested that they can reduces the disease propagation between cells. They can also be exploited to block the pathological interaction between proteins by binding to the specific interaction sites. d Gene delivery is another potential therapeutic avenue for loss-of-function mutations. Virus can provide a functional replacement of a missing gene by mRNA or cDNA delivery. This approach was particularly tested in spinal muscular atrophy and revealed great outcomes but is not yet extensively tested in ALS [231]
At the moment, it is obvious that patients with ALS would welcome the possibility of any general treatment before having to be excluded based on their genetic status or some other criteria. However, we consider that the achievement of successful clinical trials for any treatment could be increased with sub-groups of patients established on genetic screening and biomarkers without excluding any patients. Clearly, more work has to be performed before appropriate clinical use of biomarkers but we expect that research will be improved in the next years.
Abbreviations
AAV, adeno-associated viral; ALS, amyotrophic lateral sclerosis; ASO, antisense oligonucleotide; BAC, bacterial artificial chromosome; CNS, central nervous system; CSF, cerebrospinal fluid; DM, degenerative myelopathy; DPR, dipeptide repeat protein; DTI, diffusion tensor imaging; EEC, El escorial criteria; fALS, familial amyotrophic lateral sclerosis; FTD, frontotemporal dementia; FUS, fused in sarcoma; GDNF, glial cell line-delivered neurotrophic factor; GFAP, glial fibrillary acidic protein; IGF-1, insulin growth factor 1; iPSCs, induced pluripotent stem cells; KO, knock-out; LBHI, lewy-body like hyaline inclusions; LMN, lower motor neuron; MN, motor neuron; mPrp, mouse prion promoter; MRI, magnetic resonance imaging; NCI, neuronal cytosolic inclusions; NFH, neurofilament heavy; NFL, neurofilament light; NMDA, N-methyl-D-aspartate; NMJ, neuromuscular junction; OPTN, Optineurin; PD, Parkinson’s disease; PET, positron emission tomography; PM, personalized medicine; PSP, progressive supranuclear palsy; sALS, sporadic amyotrophic lateral sclerosis; siRNA, small interfering RNA; SOD1, superoxide dismutase 1; TARDBP (TDP-43), TAR DNA-binding protein; TBK1, TANK-binding kinase 1; UBQLN2, Ubiliquin-2; UMN, upper motor neuron; VBM, voxel-based morphometry; VOC, volatile organic compounds
References
Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim Biophys Acta. 2014. doi:10.1016/j.bbadis.2014.08.010
Mancuso R, Navarro X. Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Prog Neurobiol. 2015;133:1–26. doi:10.1016/j.pneurobio.2015.07.004.
Ittner LM, Halliday GM, Kril JJ, Gotz J, Hodges JR, Kiernan MC. FTD and ALS--translating mouse studies into clinical trials. Nature reviews. Neurology. 2015;11(6):360–6. doi:10.1038/nrneurol.2015.65. Epub 2015 May 5.
Moujalled D, White AR. Advances in the Development of Disease-Modifying Treatments for Amyotrophic Lateral Sclerosis. CNS Drugs. 2016;30(3):227–43. doi:10.1007/s40263-016-0317-8.
Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73(10):805–11. doi:10.1212/WNL.0b013e3181b6bbbd.
Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688–700. doi:10.1056/nejm200105313442207.
Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10(11):661–70. doi:10.1038/nrneurol.2014.184.
Hammad M, Silva A, Glass J, Sladky JT, Benatar M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology. 2007;69(24):2236–42. doi:10.1212/01.wnl.0000286948.99150.16.
Bradley WG, Good P, Rasool CG, Adelman LS. Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann Neurol. 1983;14(3):267–77. doi:10.1002/ana.410140304.
Iglesias C, Sangari S, El Mendili MM, Benali H, Marchand-Pauvert V, Pradat PF. Electrophysiological and spinal imaging evidences for sensory dysfunction in amyotrophic lateral sclerosis. BMJ Open. 2015;5(2):e007659. doi:10.1136/bmjopen-2015-007659.
Ferguson TA, Elman LB. Clinical presentation and diagnosis of amyotrophic lateral sclerosis. NeuroRehabilitation. 2007;22(6):409–16.
Chio A, Calvo A, Moglia C, Mazzini L, Mora G. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82(7):740–6. doi:10.1136/jnnp.2010.235952.
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65(4):586–90. doi:10.1212/01.wnl.0000172911.39167.b6.
Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30(2):103–12. doi:10.1111/j.1440-1789.2009.01091.x.
Bennion Callister J, Pickering-Brown SM. Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS. Exp Neurol. 2014;262(Pt B):84–90. doi:10.1016/j.expneurol.2014.06.001.
McGuire V, Longstreth Jr WT, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology. 1996;47(2):571–3.
Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology. 1999;52(3):504–9.
Beghi E, Millul A, Micheli A, Vitelli E, Logroscino G. Incidence of ALS in Lombardy, Italy. Neurology. 2007;68(2):141–5. doi:10.1212/01.wnl.0000250339.14392.bb.
Manjaly ZR, Scott KM, Abhinav K, Wijesekera L, Ganesalingam J, Goldstein LH, Janssen A, Dougherty A, Willey E, Stanton BR, Turner MR, Ampong MA, Sakel M, Orrell RW, Howard R, Shaw CE, Leigh PN, Al-Chalabi A. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph Lateral Scler. 2010;11(5):439–42. doi:10.3109/17482961003610853.
Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–28. doi:10.1038/nrneurol.2013.203.
Testa D, Lovati R, Ferrarini M, Salmoiraghi F, Filippini G. Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28-year period. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(4):208–12.
Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, Schoenfeld DA, Hosler BA, Horvitz HR, Brown RH. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41(2):210–21. doi:10.1002/ana.410410212.
Juneja T, Pericak-Vance MA, Laing NG, Dave S, Siddique T. Prognosis in familial amyotrophic lateral sclerosis: progression and survival in patients with glu100gly and ala4val mutations in Cu, Zn superoxide dismutase. Neurology. 1997;48(1):55–7.
Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of military service and mortality from ALS. Neurology. 2005;64(1):32–7. doi:10.1212/01.wnl.0000148649.17706.d9.
Abel EL. Football increases the risk for Lou Gehrig's disease, amyotrophic lateral sclerosis. Percept Mot Skills. 2007;104(3 Pt 2):1251–4. doi:10.2466/pms.104.4.1251-1254.
Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(Pt 3):472–6. doi:10.1093/brain/awh373.
Huisman MH, Seelen M, de Jong SW, Dorresteijn KR, van Doormaal PT, van der Kooi AJ, de Visser M, Schelhaas HJ, van den Berg LH, Veldink JH. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(9):976–81. doi:10.1136/jnnp-2012-304724.
McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69(9):918–29. doi:10.1097/NEN.0b013e3181ee7d85.
Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;73(20):1693–8. doi:10.1212/WNL.0b013e3181c1df48.
O’Reilly EJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, Thun M, Park Y, Kolonel LN, Ascherio A. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):205–11. doi:10.3109/21678421.2012.735240.
Kato S. Amyotrophic lateral sclerosis models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):97–114. doi:10.1007/s00401-007-0308-4.
Saberi S, Stauffer JE, Schulte DJ, Ravits J. Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. Neurol Clin. 2015;33(4):855–76. doi:10.1016/j.ncl.2015.07.012.
Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, Gorno-Tempini ML. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology. 2005;65(1):75–80. doi:10.1212/01.wnl.0000167602.38643.29.
Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, Atkins L, Williams SC, Leigh PN. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol. 2005;252(3):321–31. doi:10.1007/s00415-005-0646-x.
Kassubek J, Unrath A, Huppertz HJ, Lule D, Ethofer T, Sperfeld AD, Ludolph AC. Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel-based morphometry of 3-D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(4):213–20. doi:10.1080/14660820510038538.
Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55(2):221–35. doi:10.1002/ana.10805.
Sta M, Sylva-Steenland RM, Casula M, de Jong JM, Troost D, Aronica E, Baas F. Innate and adaptive immunity in amyotrophic lateral sclerosis: evidence of complement activation. Neurobiol Dis. 2011;42(3):211–20. doi:10.1016/j.nbd.2011.01.002.
Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, eigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601–9. doi:10.1016/j.nbd.2003.12.012.
Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–9.
Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol. 2000;57(8):1171–6.
de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K, Mitsumoto H, Nodera H, Shefner J, Swash M. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497–503. doi:10.1016/j.clinph.2007.09.143.
Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review a systematic review. Arch Neurol. 2012;69(11):1410–6. doi:10.1001/archneurol.2012.254.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi:10.1038/362059a0.
Eisen A, Mezei MM, Stewart HG, Fabros M, Gibson G, Andersen PM. SOD1 gene mutations in ALS patients from British Columbia, Canada: clinical features, neurophysiology and ethical issues in management. Amyotroph Lateral Scler. 2008;9(2):108–19. doi:10.1080/17482960801900073.
Andersen PM, Forsgren L, Binzer M, Nilsson P, Ala-Hurula V, Keranen ML, Bergmark L, Saarinen A, Haltia T, Tarvainen I, Kinnunen E, Udd B, Marklund SL. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain. 1996;119(Pt 4):1153–72.
Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, Guillot-Noel L, Russaouen O, Bruneteau G, Pradat PF, Le Forestier N, Vandenberghe N, Danel-Brunaud V, Guy N, Thauvin-Robinet C, Lacomblez L, Couratier P, Hannequin D, Seilhean D, Le Ber I, Corcia P, Camu W, Brice A, Rouleau G, LeGuern E, Meininger V. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J Med Genet. 2010;47(8):554–60. doi:10.1136/jmg.2010.077180.
Brotherton T, Polak M, Kelly C, Birve A, Andersen P, Marklund SL, Glass JD. A novel ALS SOD1 C6S mutation with implications for aggregation related toxicity and genetic counseling. Amyotroph Lateral Scler. 2011;12(3):215–9. doi:10.3109/17482968.2010.531279.
Battistini S, Giannini F, Greco G, Bibbo G, Ferrera L, Marini V, Causarano R, Casula M, Lando G, Patrosso MC, Caponnetto C, Origone P, Marocchi A, Del Corona A, Siciliano G, Carrera P, Mascia V, Giagheddu M, Carcassi C, Orru S, Garre C, Penco S. SOD1 mutations in amyotrophic lateral sclerosis. Results from a multicenter Italian study. J Neurol. 2005;252(7):782–8. doi:10.1007/s00415-005-0742-y.
Mase G, Ros S, Gemma A, Bonfigli L, Carraro N, Cazzato G, Rolfo M, Zanconati F, Sepcic J, Jurjevic A, Pirulli D, Boniotto M, Zezlina S, Crovella S, Amoroso A. ALS with variable phenotypes in a six-generation family caused by leu144phe mutation in the SOD1 gene. J Neurol Sci. 2001;191(1-2):11–8.
Katz JS, Katzberg HD, Woolley SC, Marklund SL, Andersen PM. Combined fulminant frontotemporal dementia and amyotrophic lateral sclerosis associated with an I113T SOD1 mutation. Amyotroph Lateral Scler. 2012;13(6):567–9. doi:10.3109/17482968.2012.678365.
Nakamura M, Bieniek KF, Lin WL, Graff-Radford NR, Murray ME, Castanedes-Casey M, Desaro P, Baker MC, Rutherford NJ, Robertson J, Rademakers R, Dickson DW, Boylan KB. A truncating SOD1 mutation, p.Gly141X, is associated with clinical and pathologic heterogeneity, including frontotemporal lobar degeneration. Acta Neuropathol. 2015;130(1):145–57. doi:10.1007/s00401-015-1431-2.
Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7(11):603–15. doi:10.1038/nrneurol.2011.150.
Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY, Kato T, Asayama K. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol. 1996;55(4):481–90.
Inoue K, Fujimura H, Ogawa Y, Satoh T, Shimada K, Sakoda S. Familial amyotrophic lateral sclerosis with a point mutation (G37R) of the superoxide dismutase 1 gene: a clinicopathological study. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3(4):244–7.
Ohi T, Nabeshima K, Kato S, Yazawa S, Takechi S. Familial amyotrophic lateral sclerosis with His46Arg mutation in Cu/Zn superoxide dismutase presenting characteristic clinical features and Lewy body-like hyaline inclusions. J Neurol Sci. 2004;225(1-2):19–25. doi:10.1016/j.jns.2004.06.008.
Shaw CE, Enayat ZE, Powell JF, Anderson VE, Radunovic A, Al-Sarraj S, Leigh PN. Familial amyotrophic lateral sclerosis. Molecular pathology of a patient with a SOD1 mutation. Neurology. 1997;49(6):1612–6.
Orrell RW, King AW, Hilton DA, Campbell MJ, Lane RJ, de Belleroche JS. Familial amyotrophic lateral sclerosis with a point mutation of SOD-1: intrafamilial heterogeneity of disease duration associated with neurofibrillary tangles. J Neurol Neurosurg Psychiatry. 1995;59(3):266–70.
Takehisa Y, Ujike H, Ishizu H, Terada S, Haraguchi T, Tanaka Y, Nishinaka T, Nobukuni K, Ihara Y, Namba R, Yasuda T, Nishibori M, Hayabara T, Kuroda S. Familial amyotrophic lateral sclerosis with a novel Leu126Ser mutation in the copper/zinc superoxide dismutase gene showing mild clinical features and lewy body-like hyaline inclusions. Arch Neurol. 2001;58(5):736–40.
Kato S, Takikawa M, Nakashima K, Hirano A, Cleveland DW, Kusaka H, Shibata N, Kato M, Nakano I, Ohama E. New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (SOD1) gene mutations: inclusions containing SOD1 in neurons and astrocytes. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(3):163–84.
Kato S, Shimoda M, Watanabe Y, Nakashima K, Takahashi K, Ohama E. Familial amyotrophic lateral sclerosis with a two base pair deletion in superoxide dismutase 1: gene multisystem degeneration with intracytoplasmic hyaline inclusions in astrocytes. J Neuropathol Exp Neurol. 1996;55(10):1089–101.
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–5.
Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20(7):2534–42.
Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7(8):1427–31.
Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185(2):232–40.
Gowing G, Philips T, Van Wijmeersch B, Audet JN, Dewil M, Van Den Bosch L, Billiau AD, Robberecht W, Julien JP. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J Neurosci. 2008;28(41):10234–44. doi:10.1523/jneurosci.3494-08.2008.
Brownell AL, Kuruppu D, Kil KE, Jokivarsi K, Poutiainen P, Zhu A, Maxwell M. PET imaging studies show enhanced expression of mGluR5 and inflammatory response during progressive degeneration in ALS mouse model expressing SOD1-G93A gene. J Neuroinflammation. 2015;12(1):217. doi:10.1186/s12974-015-0439-9.
Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr., Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13(1):43–7. doi:10.1038/ng0596-43.
Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, Siwek DF, Upton-Rice M, Brown RH, Jr. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53(6):1239–46.
Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, London J, Holstege JC. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7(6 Pt B):623–43. doi:10.1006/nbdi.2000.0299.
Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O'Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103(18):7142–7. doi:10.1073/pnas.0602046103.
Graffmo KS, Forsberg K, Bergh J, Birve A, Zetterstrom P, Andersen PM, Marklund SL, Brannstrom T. Expression of wild-type human superoxide dismutase-1 in mice causes amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22(1):51–60. doi:10.1093/hmg/dds399.
Pfohl SR, Halicek MT, Mitchell CS. Characterization of the Contribution of Genetic Background and Gender to Disease Progression in the SOD1 G93A Mouse Model of Amyotrophic Lateral Sclerosis: A Meta-Analysis. J Neuromuscul Dis. 2015;2(2):137–50. doi:10.3233/jnd-140068.
McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7(6):557–70. doi:10.1016/j.genm.2010.11.010.
Siddique T, Deng HX. Genetics of amyotrophic lateral sclerosis. Hum Mol Genet. 1996;5(Spec No):1465–70.
Jonsson PA, Graffmo KS, Brannstrom T, Nilsson P, Andersen PM, Marklund SL. Motor neuron disease in mice expressing the wild type-like D90A mutant superoxide dismutase-1. J Neuropathol Exp Neurol. 2006;65(12):1126–36. doi:10.1097/01.jnen.0000248545.36046.3c.
Filali M, Lalonde R, Rivest S. Sensorimotor and cognitive functions in a SOD1(G37R) transgenic mouse model of amyotrophic lateral sclerosis. Behav Brain Res. 2011;225(1):215–21. doi:10.1016/j.bbr.2011.07.034.
Quarta E, Bravi R, Scambi I, Mariotti R, Minciacchi D. Increased anxiety-like behavior and selective learning impairments are concomitant to loss of hippocampal interneurons in the presymptomatic SOD1(G93A) ALS mouse model. J Comp Neurol. 2015;523(11):1622–38. doi:10.1002/cne.23759.
Joyce PI, McGoldrick P, Saccon RA, Weber W, Fratta P, West SJ, Zhu N, Carter S, Phatak V, Stewart M, Simon M, Kumar S, Heise I, Bros-Facer V, Dick J, Corrochano S, Stanford MJ, Luong TV, Nolan PM, Meyer T, Brandner S, Bennett DL, Ozdinler PH, Greensmith L, Fisher EM, Acevedo-Arozena A. A novel SOD1-ALS mutation separates central and peripheral effects of mutant SOD1 toxicity. Hum Mol Genet. 2015;24(7):1883–97. doi:10.1093/hmg/ddu605.
Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28(9):2075–88. doi:10.1523/jneurosci.5258-07.2008.
Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21(10):3369–74.
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251–3. doi:10.1038/nn2047.
Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20(2):660–5.
Kashlan ON, Kashlan BN, Oh SS, McGinley LM, Chen KS, Kupfer R, Erman A, Sakowski SA, Feldman EL. Histological Bulbar Manifestations in the ALS Rat. Neurodegener Dis. 2015;15(2):121–6. doi:10.1159/000377725.
Nardone R, Holler Y, Taylor AC, Lochner P, Tezzon F, Golaszewski S, Brigo F, Trinka E. Canine degenerative myelopathy: a model of human amyotrophic lateral sclerosis. Zoology (Jena). 2015. doi:10.1016/j.zool.2015.09.003
Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, Perloski M, Biagi T, Baranowska I, Long S, March PA, Olby NJ, Shelton GD, Khan S, O'Brien DP, Lindblad-Toh K, Coates JR. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2009;106(8):2794–9. doi:10.1073/pnas.0812297106.
Wininger FA, Zeng R, Johnson GS, Katz ML, Johnson GC, Bush WW, Jarboe JM, Coates JR. Degenerative myelopathy in a Bernese Mountain Dog with a novel SOD1 missense mutation. J Vet Intern Med. 2011;25(5):1166–70. doi:10.1111/j.1939-1676.2011.0760.x.
Ogawa M, Uchida K, Yamato O, Inaba M, Uddin MM, Nakayama H. Neuronal loss and decreased GLT-1 expression observed in the spinal cord of Pembroke Welsh Corgi dogs with canine degenerative myelopathy. Vet Pathol. 2014;51(3):591–602. doi:10.1177/0300985813495899.
Morgan BR, Coates JR, Johnson GC, Bujnak AC, Katz ML. Characterization of intercostal muscle pathology in canine degenerative myelopathy: a disease model for amyotrophic lateral sclerosis. J Neurosci Res. 2013;91(12):1639–50. doi:10.1002/jnr.23287.
Babin PJ, Goizet C, Raldua D. Zebrafish models of human motor neuron diseases: advantages and limitations. Prog Neurobiol. 2014;118:36–58. doi:10.1016/j.pneurobio.2014.03.001.
Lemmens R, Van Hoecke A, Hersmus N, Geelen V, D'Hollander I, Thijs V, Van Den Bosch L, Carmeliet P, Robberecht W. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum Mol Genet. 2007;16(19):2359–65. doi:10.1093/hmg/ddm193.
Sakowski SA, Lunn JS, Busta AS, Oh SS, Zamora-Berridi G, Palmer M, Rosenberg AA, Philip SG, Dowling JJ, Feldman EL. Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol Neurodegener. 2012;7:44. doi:10.1186/1750-1326-7-44.
Ramesh T, Lyon AN, Pineda RH, Wang C, Janssen PM, Canan BD, Burghes AH, Beattie CE. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech. 2010;3(9-10):652–62. doi:10.1242/dmm.005538.
Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem. 2008;283(36):24972–81. doi:10.1074/jbc.M804817200.
Brignull HR, Morley JF, Morimoto RI. The stress of misfolded proteins: C. elegans models for neurodegenerative disease and aging. Adv Exp Med Biol. 2007;594:167–89. doi:10.1007/978-0-387-39975-1_15.
Ogawa M, Shidara H, Oka K, Kurosawa M, Nukina N, Furukawa Y. Cysteine residues in Cu, Zn-superoxide dismutase are essential to toxicity in Caenorhabditis elegans model of amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2015;463(4):1196–202. doi:10.1016/j.bbrc.2015.06.084.
Bjarkam CR, Nielsen MS, Glud AN, Rosendal F, Mogensen P, Bender D, Doudet D, Moller A, Sorensen JC. Neuromodulation in a minipig MPTP model of Parkinson disease. Br J Neurosurg. 2008;22 Suppl 1:S9–12. doi:10.1080/02688690802448285.
Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bogh IB, Holm IE, Jakobsen JE, Johansen MG, Purup S, Bolund L, Vajta G, Jorgensen AL. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer's disease-causing dominant mutation APPsw. Transgenic Res. 2009;18(4):545–58. doi:10.1007/s11248-009-9245-4.
Uchida M, Shimatsu Y, Onoe K, Matsuyama N, Niki R, Ikeda JE, Imai H. Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res. 2001;10(6):577–82.
Yang H, Wang G, Sun H, Shu R, Liu T, Wang CE, Liu Z, Zhao Y, Zhao B, Ouyang Z, Yang D, Huang J, Zhou Y, Li S, Jiang X, Xiao Z, Li XJ, Lai L. Species-dependent neuropathology in transgenic SOD1 pigs. Cell Res. 2014;24(4):464–81. doi:10.1038/cr.2014.25.
Jiang H, Wang C, Ren M, Yin X, Chi C, Guo L, Ke C, Feng H, Li E. Blood volatile organic compounds as potential biomarkers for amyotrophic lateral sclerosis: an animal study in the SOD1 G93A mouse. J Mol Neurosci. 2015;55(1):167–73. doi:10.1007/s12031-014-0297-4.
Toivonen JM, Manzano R, Olivan S, Zaragoza P, Garcia-Redondo A, Osta R. MicroRNA-206: a potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS One. 2014;9(2):e89065. doi:10.1371/journal.pone.0089065.
Freischmidt A, Muller K, Zondler L, Weydt P, Volk AE, Bozic AL, Walter M, Bonin M, Mayer B, von Arnim CA, Otto M, Dieterich C, Holzmann K, Andersen PM, Ludolph AC, Danzer KM, Weishaupt JH. Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain. 2014;137(Pt 11):2938–50. doi:10.1093/brain/awu249.
Caron I, Micotti E, Paladini A, Merlino G, Plebani L, Forloni G, Modo M, Bendotti C. Comparative Magnetic Resonance Imaging and Histopathological Correlates in Two SOD1 Transgenic Mouse Models of Amyotrophic Lateral Sclerosis. PLoS One. 2015;10(7):e0132159. doi:10.1371/journal.pone.0132159.
Evans MC, Serres S, Khrapitchev AA, Stolp HB, Anthony DC, Talbot K, Turner MR, Sibson NR. T(2)-weighted MRI detects presymptomatic pathology in the SOD1 mouse model of ALS. J Cereb Blood Flow Metab. 2014;34(5):785–93. doi:10.1038/jcbfm.2014.19.
Schleidgen S, Klingler C, Bertram T, Rogowski WH, Marckmann G. What is personalized medicine: sharpening a vague term based on a systematic literature review. BMC Med Ethics. 2013;14:55. doi:10.1186/1472-6939-14-55.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi:10.1056/NEJMoa1103782.
Robertson J, Sanelli T, Xiao S, Yang W, Horne P, Hammond R, Pioro EP, Strong MJ. Lack of TDP-43 abnormalities in mutant SOD1 transgenic mice shows disparity with ALS. Neurosci Lett. 2007;420(2):128–32. doi:10.1016/j.neulet.2007.03.066.
Shan X, Vocadlo D, Krieger C. Mislocalization of TDP-43 in the G93A mutant SOD1 transgenic mouse model of ALS. Neurosci Lett. 2009;458(2):70–4. doi:10.1016/j.neulet.2009.04.031.
Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113(5):535–42. doi:10.1007/s00401-007-0206-9.
Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–42. doi:10.1126/science.1086137.
Patel P, Kriz J, Gravel M, Soucy G, Bareil C, Gravel C, Julien JP. Adeno-associated virus-mediated delivery of a recombinant single-chain antibody against misfolded superoxide dismutase for treatment of amyotrophic lateral sclerosis. Mol Ther. 2014;22(3):498–510. doi:10.1038/mt.2013.239.
Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11(4):429–33. doi:10.1038/nm1205.
Thomsen GM, Gowing G, Latter J, Chen M, Vit JP, Staggenborg K, Avalos P, Alkaslasi M, Ferraiuolo L, Likhite S, Kaspar BK, Svendsen CN. Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J Neurosci. 2014;34(47):15587–600. doi:10.1523/jneurosci.2037-14.2014.
Foust KD, Salazar DL, Likhite S, Ferraiuolo L, Ditsworth D, Ilieva H, Meyer K, Schmelzer L, Braun L, Cleveland DW, Kaspar BK. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Molecular therapy: The Journal of the American Society of Gene Therapy. 2013;21(12):2148–59. doi:10.1038/mt.2013.211.
Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, Hung G, Lobsiger CS, Ward CM, McAlonis-Downes M, Wei H, Wancewicz EV, Bennett CF, Cleveland DW. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116(8):2290–6. doi:10.1172/jci25424.
Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K, Ostrow LW, Schoenfeld D, Macklin EA, Norris DA, Manousakis G, Crisp M, Smith R, Bennett CF, Bishop KM, Cudkowicz ME. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12(5):435–42. doi:10.1016/s1474-4422(13)70061-9.
Ratti A, Buratti E. Physiological Functions and Pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem. 2016. doi:10.1111/jnc.13625
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. In: Science, vol 314. vol 5796. United States: 2006. pp 130-133. doi:10.1126/science.1134108
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–11. doi:10.1016/j.bbrc.2006.10.093.
Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, 3rd, Bigio EH, Caselli R, Baker M, Al-Lozi MT, Morris JC, Pestronk A, Rademakers R, Goate AM, Cairns NJ. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63(4):535–8. doi:10.1002/ana.21344.
Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–4. doi:10.1038/ng.132.
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–72. doi:10.1126/science.1154584.
Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7(5):409–16. doi:10.1016/s1474-4422(08)70071-1.
Corcia P, Valdmanis P, Millecamps S, Lionnet C, Blasco H, Mouzat K, Daoud H, Belzil V, Morales R, Pageot N, Danel-Brunaud V, Vandenberghe N, Pradat PF, Couratier P, Salachas F, Lumbroso S, Rouleau GA, Meininger V, Camu W. Phenotype and genotype analysis in amyotrophic lateral sclerosis with TARDBP gene mutations. Neurology. 2012;78(19):1519–26. doi:10.1212/WNL.0b013e3182553c88.
Yamashita S, Ando Y. Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener. 2015;4:13. doi:10.1186/s40035-015-0036-y.
Floris G, Borghero G, Cannas A, Di Stefano F, Murru MR, Corongiu D, Cuccu S, Tranquilli S, Cherchi MV, Serra A, Loi G, Marrosu MG, Chio A, Marrosu F. Clinical phenotypes and radiological findings in frontotemporal dementia related to TARDBP mutations. J Neurol. 2015;262(2):375–84. doi:10.1007/s00415-014-7575-5.
Chio A, Calvo A, Moglia C, Restagno G, Ossola I, Brunetti M, Montuschi A, Cistaro A, Ticca A, Traynor BJ, Schymick JC, Mutani R, Marrosu MG, Murru MR, Borghero G. Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations. Arch Neurol. 2010;67(8):1002–9. doi:10.1001/archneurol.2010.173.
Quadri M, Cossu G, Saddi V, Simons EJ, Murgia D, Melis M, Ticca A, Oostra BA, Bonifati V. Broadening the phenotype of TARDBP mutations: the TARDBP Ala382Thr mutation and Parkinson's disease in Sardinia. Neurogenetics. 2011;12(3):203–9. doi:10.1007/s10048-011-0288-3.
Synofzik M, Born C, Rominger A, Lummel N, Schols L, Biskup S, Schule C, Grasshoff U, Klopstock T, Adamczyk C. Targeted high-throughput sequencing identifies a TARDBP mutation as a cause of early-onset FTD without motor neuron disease. Neurobiol Aging. 2014;35(5):1212.e1211–1215. doi:10.1016/j.neurobiolaging.2013.10.092.
Chio A, Borghero G, Pugliatti M, Ticca A, Calvo A, Moglia C, Mutani R, Brunetti M, Ossola I, Marrosu MG, Murru MR, Floris G, Cannas A, Parish LD, Cossu P, Abramzon Y, Johnson JO, Nalls MA, Arepalli S, Chong S, Hernandez DG, Traynor BJ, Restagno G. Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch Neurol. 2011;68(5):594–8. doi:10.1001/archneurol.2010.352.
Okamoto K, Fujita Y, Hoshino E, Tamura Y, Fukuda T, Hasegawa M, Takatama M. An autopsy case of familial amyotrophic lateral sclerosis with a TARDBP Q343R mutation. Neuropathology. 2015;35(5):462–8. doi:10.1111/neup.12209.
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171(1):227–40. doi:10.2353/ajpath.2007.070182.
Swarup V, Phaneuf D, Dupre N, Petri S, Strong M, Kriz J, Julien JP. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor kappaB-mediated pathogenic pathways. In: J Exp Med, vol 208. vol 12. United States: 2011. pp 2429-2447. doi:10.1084/jem.20111313
Stallings NR, Puttaparthi K, Luther CM, Burns DK, Elliott JL. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol Dis. 2010;40(2):404–14. doi:10.1016/j.nbd.2010.06.017.
Xu YF, Gendron TF, Zhang YJ, Lin WL, D'Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J, Petrucelli L. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci. 2010;30(32):10851–9. doi:10.1523/jneurosci.1630-10.2010.
Esmaeili MA, Panahi M, Yadav S, Hennings L, Kiaei M. Premature death of TDP-43 (A315T) transgenic mice due to gastrointestinal complications prior to development of full neurological symptoms of amyotrophic lateral sclerosis. Int J Exp Pathol. 2013;94(1):56–64. doi:10.1111/iep.12006.
Hatzipetros T, Bogdanik LP, Tassinari VR, Kidd JD, Moreno AJ, Davis C, Osborne M, Austin A, Vieira FG, Lutz C, Perrin S. C57BL/6J congenic Prp-TDP43A315T mice develop progressive neurodegeneration in the myenteric plexus of the colon without exhibiting key features of ALS. Brain Res. 2014;1584:59–72. doi:10.1016/j.brainres.2013.10.013.
Swarup V, Phaneuf D, Bareil C, Robertson J, Rouleau GA, Kriz J, Julien JP. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011;134(Pt 9):2610–26. doi:10.1093/brain/awr159.
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. In: Proc Natl Acad Sci U S A, vol 106. vol 44. United States: 2009. pp 18809-18814. doi:10.1073/pnas.0908767106
Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, Da Cruz S, Clutario KM, Swing D, Tessarollo L, Marsala M, Shaw CE, Yeo GW, Cleveland DW. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013;110(8):E736–45. doi:10.1073/pnas.1222809110.
Mitchell JC, Constable R, So E, Vance C, Scotter E, Glover L, Hortobagyi T, Arnold ES, Ling SC, McAlonis M, Da Cruz S, Polymenidou M, Tessarolo L, Cleveland DW, Shaw CE. Wild type human TDP-43 potentiates ALS-linked mutant TDP-43 driven progressive motor and cortical neuron degeneration with pathological features of ALS. Acta Neuropathol Commun. 2015;3:36. doi:10.1186/s40478-015-0212-4.
Zhou H, Huang C, Chen H, Wang D, Landel CP, Xia PY, Bowser R, Liu YJ, Xia XG. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6(3):e1000887. doi:10.1371/journal.pgen.1000887.
Huang C, Tong J, Bi F, Zhou H, Xia XG. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J Clin Invest. 2012;122(1):107–18. doi:10.1172/jci59130.
Casci I, Pandey UB. A fruitful endeavor: modeling ALS in the fruit fly. Brain Res. 2015;1607:47–74. doi:10.1016/j.brainres.2014.09.064.
Hanson KA, Kim SH, Wassarman DA, Tibbetts RS. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS). J Biol Chem. 2010;285(15):11068–72. doi:10.1074/jbc.C109.078527.
Voigt A, Herholz D, Fiesel FC, Kaur K, Muller D, Karsten P, Weber SS, Kahle PJ, Marquardt T, Schulz JB. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS One. 2010;5(8):e12247. doi:10.1371/journal.pone.0012247.
Cheng CW, Lin MJ, Shen CK. Rapamycin alleviates pathogenesis of a new Drosophila model of ALS-TDP. J Neurogenet. 2015;29(2-3):59–68. doi:10.3109/01677063.2015.1077832.
Lin MJ, Cheng CW, Shen CK. Neuronal function and dysfunction of Drosophila dTDP. PLoS One. 2011;6(6):e20371. doi:10.1371/journal.pone.0020371.
Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD. Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet. 2010;19(16):3206–18. doi:10.1093/hmg/ddq230.
Liachko NF, Guthrie CR, Kraemer BC. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J Neurosci. 2010;30(48):16208–19. doi:10.1523/jneurosci.2911-10.2010.
Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, Rochefort D, Bel Hadj S, Durham HD, Vande Velde C, Rouleau GA, Drapeau P. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet. 2010;19(4):671–83. doi:10.1093/hmg/ddp534.
Laird AS, Van Hoecke A, De Muynck L, Timmers M, Van den Bosch L, Van Damme P, Robberecht W. Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PLoS One. 2010;5(10):e13368. doi:10.1371/journal.pone.0013368.
Verstraete E, Kuiperij HB, van Blitterswijk MM, Veldink JH, Schelhaas HJ, van den Berg LH, Verbeek MM. TDP-43 plasma levels are higher in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(5):446–51. doi:10.3109/17482968.2012.703208.
Kasai T, Tokuda T, Ishigami N, Sasayama H, Foulds P, Mitchell DJ, Mann DM, Allsop D, Nakagawa M. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2009;117(1):55–62. doi:10.1007/s00401-008-0456-1.
Noto Y, Shibuya K, Sato Y, Kanai K, Misawa S, Sawai S, Mori M, Uchiyama T, Isose S, Nasu S, Sekiguchi Y, Fujimaki Y, Kasai T, Tokuda T, Nakagawa M, Kuwabara S. Elevated CSF TDP-43 levels in amyotrophic lateral sclerosis: specificity, sensitivity, and a possible prognostic value. Amyotroph Lateral Scler. 2011;12(2):140–3. doi:10.3109/17482968.2010.541263.
Pare B, Touzel-Deschenes L, Lamontagne R, Lamarre MS, Scott FD, Khuong HT, Dion PA, Bouchard JP, Gould P, Rouleau GA, Dupre N, Berthod F, Gros-Louis F. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol Commun. 2015;3:5. doi:10.1186/s40478-014-0181-z.
Nishimura AL, Shum C, Scotter EL, Abdelgany A, Sardone V, Wright J, Lee YB, Chen HJ, Bilican B, Carrasco M, Maniatis T, Chandran S, Rogelj B, Gallo JM, Shaw CE. Allele-specific knockdown of ALS-associated mutant TDP-43 in neural stem cells derived from induced pluripotent stem cells. PLoS One. 2014;9(3):e91269. doi:10.1371/journal.pone.0091269.
Jackson KL, Dayton RD, Orchard EA, Ju S, Ringe D, Petsko GA, Maquat LE, Klein RL. Preservation of forelimb function by UPF1 gene therapy in a rat model of TDP-43-induced motor paralysis. Gene Ther. 2015;22(1):20–8. doi:10.1038/gt.2014.101.
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68. doi:10.1016/j.neuron.2011.09.010.
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–56. doi:10.1016/j.neuron.2011.09.011.
Suh E, Lee EB, Neal D, Wood EM, Toledo JB, Rennert L, Irwin DJ, McMillan CT, Krock B, Elman LB, McCluskey LF, Grossman M, Xie SX, Trojanowski JQ, Van Deerlin VM. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol. 2015;130(3):363–72. doi:10.1007/s00401-015-1445-9.
Millecamps S, Boillee S, Le Ber I, Seilhean D, Teyssou E, Giraudeau M, Moigneu C, Vandenberghe N, Danel-Brunaud V, Corcia P, Pradat PF, Le Forestier N, Lacomblez L, Bruneteau G, Camu W, Brice A, Cazeneuve C, Leguern E, Meininger V, Salachas F. Phenotype difference between ALS patients with expanded repeats in C9ORF72 and patients with mutations in other ALS-related genes. J Med Genet. 2012;49(4):258–63. doi:10.1136/jmedgenet-2011-100699.
Debray S, Race V, Crabbe V, Herdewyn S, Matthijs G, Goris A, Dubois B, Thijs V, Robberecht W, Van Damme P. Frequency of C9orf72 repeat expansions in amyotrophic lateral sclerosis: a Belgian cohort study. Neurobiol Aging. 2013;34(12):2890.e2897–12. doi:10.1016/j.neurobiolaging.2013.06.009.
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, McLaughlin RL, Iyer PM, O'Brien C, Phukan J, Wynne B, Bokde AL, Bradley DG, Pender N, Al-Chalabi A, Hardiman O. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11(3):232–40. doi:10.1016/s1474-4422(12)70014-5.
Chio A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, Sendtner M, Brunetti M, Ossola I, Calvo A, Pugliatti M, Sotgiu MA, Murru MR, Marrosu MG, Marrosu F, Marinou K, Mandrioli J, Sola P, Caponnetto C, Mancardi G, Mandich P, La Bella V, Spataro R, Conte A, Monsurro MR, Tedeschi G, Pisano F, Bartolomei I, Salvi F, Lauria Pinter G, Simone I, Logroscino G, Gambardella A, Quattrone A, Lunetta C, Volanti P, Zollino M, Penco S, Battistini S, Renton AE, Majounie E, Abramzon Y, Conforti FL, Giannini F, Corbo M, Sabatelli M. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain. 2012;135(Pt 3):784–93. doi:10.1093/brain/awr366.
Snowden JS, Adams J, Harris J, Thompson JC, Rollinson S, Richardson A, Jones M, Neary D, Mann DM, Pickering-Brown S. Distinct clinical and pathological phenotypes in frontotemporal dementia associated with MAPT, PGRN and C9orf72 mutations. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(7-8):497–505. doi:10.3109/21678421.2015.1074700.
Wilke C, Pomper JK, Biskup S, Puskas C, Berg D, Synofzik M. Atypical parkinsonism in C9orf72 expansions: a case report and systematic review of 45 cases from the literature. J Neurol. 2016. doi:10.1007/s00415-016-8021-7
Akimoto C, Forsgren L, Linder J, Birve A, Backlund I, Andersson J, Nilsson AC, Alstermark H, Andersen PM. No GGGGCC-hexanucleotide repeat expansion in C9ORF72 in parkinsonism patients in Sweden. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(1):26–9. doi:10.3109/17482968.2012.725415.
Majounie E, Abramzon Y, Renton AE, Keller MF, Traynor BJ, Singleton AB. Large C9orf72 repeat expansions are not a common cause of Parkinson's disease. Neurobiol Aging. 2012;33(10):2527.e2521–2522. doi:10.1016/j.neurobiolaging.2012.05.007.
Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, Restagno G, Brunetti M, Traynor BJ, Nobili F, Carrara G, Fania P, Lopiano L, Valentini MC, Chio A. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging. 2014;41(5):844–52. doi:10.1007/s00259-013-2667-5.
Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman T, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122(6):673–90. doi:10.1007/s00401-011-0907-y.
Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122(6):691–702. doi:10.1007/s00401-011-0911-2.
Schipper LJ, Raaphorst J, Aronica E, Baas F, de Haan R, de Visser M, Troost D. Prevalence of brain and spinal cord inclusions, including dipeptide repeat proteins, in patients with the C9ORF72 hexanucleotide repeat expansion: A systematic neuropathological review. Neuropathol Appl Neurobiol. 2015. doi:10.1111/nan.12284
Hukema RK, Riemslagh FW, Melhem S, van der Linde HC, Severijnen LA, Edbauer D, Maas A, Charlet-Berguerand N, Willemsen R, van Swieten JC. A new inducible transgenic mouse model for C9orf72-associated GGGGCC repeat expansion supports a gain-of-function mechanism in C9orf72-associated ALS and FTD. In: Acta Neuropathol Commun, vol 2. England: 2014. p 166. doi:10.1186/s40478-014-0166-y.
Koppers M, Blokhuis AM, Westeneng HJ, Terpstra ML, Zundel CA, Vieira de Sa R, Schellevis RD, Waite AJ, Blake DJ, Veldink JH, van den Berg LH, Pasterkamp RJ. C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol. 2015;78(3):426–38. doi:10.1002/ana.24453.
Chew J, Gendron TF, Prudencio M, Sasaguri H, Zhang YJ, Castanedes-Casey M, Lee CW, Jansen-West K, Kurti A, Murray ME, Bieniek KF, Bauer PO, Whitelaw EC, Rousseau L, Stankowski JN, Stetler C, Daughrity LM, Perkerson EA, Desaro P, Johnston A, Overstreet K, Edbauer D, Rademakers R, Boylan KB, Dickson DW, Fryer JD, Petrucelli L. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science. 2015;348(6239):1151–4. doi:10.1126/science.aaa9344.
Peters OM, Cabrera GT, Tran H, Gendron TF, McKeon JE, Metterville J, Weiss A, Wightman N, Salameh J, Kim J, Sun H, Boylan KB, Dickson D, Kennedy Z, Lin Z, Zhang YJ, Daughrity L, Jung C, Gao FB, Sapp PC, Horvitz HR, Bosco DA, Brown SP, de Jong P, Petrucelli L, Mueller C, Brown RH, Jr. Human C9ORF72 Hexanucleotide Expansion Reproduces RNA Foci and Dipeptide Repeat Proteins but Not Neurodegeneration in BAC Transgenic Mice. Neuron. 2015;88(5):902–9. doi:10.1016/j.neuron.2015.11.018.
O'Rourke JG, Bogdanik L, Muhammad AK, Gendron TF, Kim KJ, Austin A, Cady J, Liu EY, Zarrow J, Grant S, Ho R, Bell S, Carmona S, Simpkinson M, Lall D, Wu K, Daughrity L, Dickson DW, Harms MB, Petrucelli L, Lee EB, Lutz CM, Baloh RH. C9orf72 BAC Transgenic Mice Display Typical Pathologic Features of ALS/FTD. Neuron. 2015;88(5):892–901. doi:10.1016/j.neuron.2015.10.027.
Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis-Downes M, Seelman A, Stauffer JE, Jafar-Nejad P, Drenner K, Schulte D, Chun S, Sun S, Ling SC, Myers B, Engelhardt J, Katz M, Baughn M, Platoshyn O, Marsala M, Watt A, Heyser CJ, Ard MC, De Muynck L, Daughrity LM, Swing DA, Tessarollo L, Jung CJ, Delpoux A, Utzschneider DT, Hedrick SM, de Jong PJ, Edbauer D, Van Damme P, Petrucelli L, Shaw CE, Bennett CF, Da Cruz S, Ravits J, Rigo F, Cleveland DW, Lagier-Tourenne C. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron. 2016;90(3):535–50. doi:10.1016/j.neuron.2016.04.006.
Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, Yachnis AT, Ranum LP. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 2016;90(3):521–34. doi:10.1016/j.neuron.2016.04.005.
Tran H, Almeida S, Moore J, Gendron TF, Chalasani U, Lu Y, Du X, Nickerson JA, Petrucelli L, Weng Z, Gao FB. Differential Toxicity of Nuclear RNA Foci versus Dipeptide Repeat Proteins in a Drosophila Model of C9ORF72 FTD/ALS. Neuron. 2015;87(6):1207–14. doi:10.1016/j.neuron.2015.09.015.
Mizielinska S, Gronke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IO, Pietrzyk J, Cleverley K, Nicoll AJ, Pickering-Brown S, Dols J, Cabecinha M, Hendrich O, Fratta P, Fisher EM, Partridge L, Isaacs AM. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345(6201):1192–4. doi:10.1126/science.1256800.
Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, Jin P. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci U S A. 2013;110(19):7778–83. doi:10.1073/pnas.1219643110.
Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, Badders N, Valentine M, Miller BL, Wong PC, Petrucelli L, Kim HJ, Gao FB, Taylor JP. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525(7567):129–33. doi:10.1038/nature14974.
Therrien M, Rouleau GA, Dion PA, Parker JA. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS One. 2013;8(12):e83450. doi:10.1371/journal.pone.0083450.
Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A, Kabashi E. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol. 2013;74(2):180–7. doi:10.1002/ana.23946.
Mendez EF, Sattler R. Biomarker development for C9orf72 repeat expansion in ALS. Brain Res. 2015;1607:26–35. doi:10.1016/j.brainres.2014.09.041.
Xi Z, Zinman L, Moreno D, Schymick J, Liang Y, Sato C, Zheng Y, Ghani M, Dib S, Keith J, Robertson J, Rogaeva E. Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am J Hum Genet. 2013;92(6):981–9. doi:10.1016/j.ajhg.2013.04.017.
Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen-West K, Belzil VV, Desaro P, Johnston A, Overstreet K, Oh SY, Todd PK, Berry JD, Cudkowicz ME, Boeve BF, Dickson D, Floeter MK, Traynor BJ, Morelli C, Ratti A, Silani V, Rademakers R, Brown RH, Rothstein JD, Boylan KB, Petrucelli L, Disney MD. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83(5):1043–50. doi:10.1016/j.neuron.2014.07.041.
Donnelly CJ, Zhang PW, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang J, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80(2):415–28. doi:10.1016/j.neuron.2013.10.015.
Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, Qiu J, Sun Y, Ling SC, Zhu Q, Polymenidou M, Drenner K, Artates JW, McAlonis-Downes M, Markmiller S, Hutt KR, Pizzo DP, Cady J, Harms MB, Baloh RH, Vandenberg SR, Yeo GW, Fu XD, Bennett CF, Cleveland DW, Ravits J. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013;110(47):E4530–9. doi:10.1073/pnas.1318835110.
Kwiatkowski Jr TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH, Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–8. doi:10.1126/science.1166066.
Corrado L, Del Bo R, Castellotti B, Ratti A, Cereda C, Penco S, Soraru G, Carlomagno Y, Ghezzi S, Pensato V, Colombrita C, Gagliardi S, Cozzi L, Orsetti V, Mancuso M, Siciliano G, Mazzini L, Comi GP, Gellera C, Ceroni M, D'Alfonso S, Silani V. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J Med Genet. 2010;47(3):190–4. doi:10.1136/jmg.2009.071027.
Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science (New York, NY). 2009;323(5918):1208–11. doi:10.1126/science.1165942.
Waibel S, Neumann M, Rosenbohm A, Birve A, Volk AE, Weishaupt JH, Meyer T, Muller U, Andersen PM, Ludolph AC. Truncating mutations in FUS/TLS give rise to a more aggressive ALS-phenotype than missense mutations: a clinico-genetic study in Germany. Eur J Neurol. 2013;20(3):540–6. doi:10.1111/ene.12031.
Lattante S, Rouleau GA, Kabashi E. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum Mutat. 2013;34(6):812–26. doi:10.1002/humu.22319.
King A, Troakes C, Smith B, Nolan M, Curran O, Vance C, Shaw CE, Al-Sarraj S. ALS-FUS pathology revisited: singleton FUS mutations and an unusual case with both a FUS and TARDBP mutation. Acta Neuropathol Commun. 2015;3:62. doi:10.1186/s40478-015-0235-x.
Hicks GG, Singh N, Nashabi A, Mai S, Bozek G, Klewes L, Arapovic D, White EK, Koury MJ, Oltz EM, Van Kaer L, Ruley HE. Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nat Genet. 2000;24(2):175–9. doi:10.1038/72842.
Kino Y, Washizu C, Kurosawa M, Yamada M, Miyazaki H, Akagi T, Hashikawa T, Doi H, Takumi T, Hicks GG, Hattori N, Shimogori T, Nukina N. FUS/TLS deficiency causes behavioral and pathological abnormalities distinct from amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2015;3:24. doi:10.1186/s40478-015-0202-6.
Mitchell JC, McGoldrick P, Vance C, Hortobagyi T, Sreedharan J, Rogelj B, Tudor EL, Smith BN, Klasen C, Miller CC, Cooper JD, Greensmith L, Shaw CE. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 2013;125(2):273–88. doi:10.1007/s00401-012-1043-z.
Sephton CF, Tang AA, Kulkarni A, West J, Brooks M, Stubblefield JJ, Liu Y, Zhang MQ, Green CB, Huber KM, Huang EJ, Herz J, Yu G. Activity-dependent FUS dysregulation disrupts synaptic homeostasis. Proc Natl Acad Sci U S A. 2014;111(44):E4769–78. doi:10.1073/pnas.1406162111.
Huang C, Zhou H, Tong J, Chen H, Liu YJ, Wang D, Wei X, Xia XG. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 2011;7(3):e1002011. doi:10.1371/journal.pgen.1002011.
Chen Y, Yang M, Deng J, Chen X, Ye Y, Zhu L, Liu J, Ye H, Shen Y, Li Y, Rao EJ, Fushimi K, Zhou X, Bigio EH, Mesulam M, Xu Q, Wu JY. Expression of human FUS protein in Drosophila leads to progressive neurodegeneration. Protein Cell. 2011;2(6):477–86. doi:10.1007/s13238-011-1065-7.
Lanson Jr NA, Maltare A, King H, Smith R, Kim JH, Taylor JP, Lloyd TE, Pandey UB. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum Mol Genet. 2011;20(13):2510–23. doi:10.1093/hmg/ddr150.
Miguel L, Avequin T, Delarue M, Feuillette S, Frebourg T, Campion D, Lecourtois M. Accumulation of insoluble forms of FUS protein correlates with toxicity in Drosophila. Neurobiol Aging. 2012;33(5):1008.e1001–1015. doi:10.1016/j.neurobiolaging.2011.10.008.
Murakami T, Yang SP, Xie L, Kawano T, Fu D, Mukai A, Bohm C, Chen F, Robertson J, Suzuki H, Tartaglia GG, Vendruscolo M, Kaminski Schierle GS, Chan FT, Moloney A, Crowther D, Kaminski CF, Zhen M, St George-Hyslop P. ALS mutations in FUS cause neuronal dysfunction and death in Caenorhabditis elegans by a dominant gain-of-function mechanism. Hum Mol Genet. 2012;21(1):1–9. doi:10.1093/hmg/ddr417.
Vaccaro A, Tauffenberger A, Aggad D, Rouleau G, Drapeau P, Parker JA. Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PLoS One. 2012;7(2):e31321. doi:10.1371/journal.pone.0031321.
Kabashi E, Bercier V, Lissouba A, Liao M, Brustein E, Rouleau GA, Drapeau P. FUS and TARDBP but not SOD1 interact in genetic models of amyotrophic lateral sclerosis. PLoS Genet. 2011;7(8):e1002214. doi:10.1371/journal.pgen.1002214.
Armstrong GA, Drapeau P. Loss and gain of FUS function impair neuromuscular synaptic transmission in a genetic model of ALS. Hum Mol Genet. 2013;22(21):4282–92. doi:10.1093/hmg/ddt278.
Kariya S, Sampson JB, Northrop LE, Luccarelli CM, Naini AB, Re DB, Hirano M, Mitsumoto H. Nuclear localization of SMN and FUS is not altered in fibroblasts from patients with sporadic ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):581–7. doi:10.3109/21678421.2014.907319.
Morlando M, Dini Modigliani S, Torrelli G, Rosa A, Di Carlo V, Caffarelli E, Bozzoni I. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. Embo j. 2012;31(24):4502–10. doi:10.1038/emboj.2012.319.
Tradewell ML, Yu Z, Tibshirani M, Boulanger MC, Durham HD, Richard S. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum Mol Genet. 2012;21(1):136–49. doi:10.1093/hmg/ddr448.
Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. In: Nature, vol 477. vol 7363. England: 2011. pp 211-215. doi:10.1038/nature10353
Millecamps S, Corcia P, Cazeneuve C, Boillee S, Seilhean D, Danel-Brunaud V, Vandenberghe N, Pradat PF, Le Forestier N, Lacomblez L, Bruneteau G, Camu W, Brice A, Meininger V, LeGuern E, Salachas F. Mutations in UBQLN2 are rare in French amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33(4):839.e831–833. doi:10.1016/j.neurobiolaging.2011.11.010.
Daoud H, Suhail H, Szuto A, Camu W, Salachas F, Meininger V, Bouchard JP, Dupre N, Dion PA, Rouleau GA. UBQLN2 mutations are rare in French and French-Canadian amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33(9):2230.e2231–5. doi:10.1016/j.neurobiolaging.2012.03.015.
Picher-Martel V, Dutta K, Phaneuf D, Sobue G, Julien JP. Ubiquilin-2 drives NF-kappaB activity and cytosolic TDP-43 aggregation in neuronal cells. Mol Brain. 2015;8(1):71. doi:10.1186/s13041-015-0162-6.
Vengoechea J, David MP, Yaghi SR, Carpenter L, Rudnicki SA. Clinical variability and female penetrance in X-linked familial FTD/ALS caused by a P506S mutation in UBQLN2. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7-8):615–9. doi:10.3109/21678421.2013.824001.
Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 2012;124(3):339–52. doi:10.1007/s00401-012-1022-4.
Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, McCluskey L, Irwin DJ, Grossman M, Molina-Porcel L, Lee VM, Trojanowski JQ. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123(6):825–39. doi:10.1007/s00401-012-0970-z.
Gorrie GH, Fecto F, Radzicki D, Weiss C, Shi Y, Dong H, Zhai H, Fu R, Liu E, Li S, Arrat H, Bigio EH, Disterhoft JF, Martina M, Mugnaini E, Siddique T, Deng HX. Dendritic spinopathy in transgenic mice expressing ALS/dementia-linked mutant UBQLN2. Proc Natl Acad Sci U S A. 2014;111(40):14524–9. doi:10.1073/pnas.1405741111.
Ceballos-Diaz C, Rosario AM, Park HJ, Chakrabarty P, Sacino A, Cruz PE, Siemienski Z, Lara N, Moran C, Ravelo N, Golde TE, McFarland NR. Viral expression of ALS-linked ubiquilin-2 mutants causes inclusion pathology and behavioral deficits in mice. Mol Neurodegener. 2015;10(1):25. doi:10.1186/s13024-015-0026-7.
Wu Q, Liu M, Huang C, Liu X, Huang B, Li N, Zhou H, Xia XG. Pathogenic Ubqln2 gains toxic properties to induce neuron death. Acta Neuropathol. 2014. doi:10.1007/s00401-014-1367-y
Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–6. doi:10.1038/nature08971.
Osawa T, Mizuno Y, Fujita Y, Takatama M, Nakazato Y, Okamoto K. Optineurin in neurodegenerative diseases. Neuropathology: Official Journal of the Japanese Society of Neuropathology. 2011;31(6):569–74. doi:10.1111/j.1440-1789.2011.01199.x.
Hortobagyi T, Troakes C, Nishimura AL, Vance C, van Swieten JC, Seelaar H, King A, Al-Sarraj S, Rogelj B, Shaw CE. Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD-TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol. 2011;121(4):519–27. doi:10.1007/s00401-011-0813-3.
Ito H, Nakamura M, Komure O, Ayaki T, Wate R, Maruyama H, Nakamura Y, Fujita K, Kaneko S, Okamoto Y, Ihara M, Konishi T, Ogasawara K, Hirano A, Kusaka H, Kaji R, Takahashi R, Kawakami H. Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol. 2011;122(2):223–9. doi:10.1007/s00401-011-0842-y.
Kamada M, Izumi Y, Ayaki T, Nakamura M, Kagawa S, Kudo E, Sako W, Maruyama H, Nishida Y, Kawakami H, Ito H, Kaji R. Clinicopathologic features of autosomal recessive amyotrophic lateral sclerosis associated with optineurin mutation. Neuropathology. 2014;34(1):64–70. doi:10.1111/neup.12051.
Bury JJ, Highley JR, Cooper-Knock J, Goodall EF, Higginbottom A, McDermott CJ, Ince PG, Shaw PJ, Kirby J. Oligogenic inheritance of optineurin (OPTN) and C9ORF72 mutations in ALS highlights localisation of OPTN in the TDP-43-negative inclusions of C9ORF72-ALS. Neuropathology. 2016;36(2):125–34. doi:10.1111/neup.12240.
Gleason CE, Ordureau A, Gourlay R, Arthur JS, Cohen P. Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon beta. J Biol Chem. 2011;286(41):35663–74. doi:10.1074/jbc.M111.267567.
Paulus JD, Link BA. Loss of optineurin in vivo results in elevated cell death and alters axonal trafficking dynamics. PLoS One. 2014;9(10):e109922. doi:10.1371/journal.pone.0109922.
Nizzardo M, Simone C, Rizzo F, Salani S, Dametti S, Rinchetti P, Del Bo R, Foust K, Kaspar BK, Bresolin N, Comi GP, Corti S. Gene therapy rescues disease phenotype in a spinal muscular atrophy with respiratory distress type 1 (SMARD1) mouse model. Sci Adv. 2015;1(2):e1500078. doi:10.1126/sciadv.1500078.
Rosen DR, Bowling AC, Patterson D, Usdin TB, Sapp P, Mezey E, McKenna-Yasek D, O'Regan J, Rahmani Z, Ferrante RJ, et al. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3(6):981–7.
Cudkowicz ME, McKenna-Yasek D, Chen C, Hedley-Whyte ET, Brown Jr RH. Limited corticospinal tract involvement in amyotrophic lateral sclerosis subjects with the A4V mutation in the copper/zinc superoxide dismutase gene. Ann Neurol. 1998;43(6):703–10. doi:10.1002/ana.410430604.
Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe K. Familial amyotrophic lateral sclerosis (ALS) in Japan associated with H46R mutation in Cu/Zn superoxide dismutase gene: a possible new subtype of familial ALS. J Neurol Sci. 1994;126(1):77–83.
Enayat ZE, Orrell RW, Claus A, Ludolph A, Bachus R, Brockmuller J, Ray-Chaudhuri K, Radunovic A, Shaw C, Wilkinson J, et al. Two novel mutations in the gene for copper zinc superoxide dismutase in UK families with amyotrophic lateral sclerosis. Hum Mol Genet. 1995;4(7):1239–40.
Aoki M, Abe K, Houi K, Ogasawara M, Matsubara Y, Kobayashi T, Mochio S, Narisawa K, Itoyama Y. Variance of age at onset in a Japanese family with amyotrophic lateral sclerosis associated with a novel Cu/Zn superoxide dismutase mutation. Ann Neurol. 1995;37(5):676–9. doi:10.1002/ana.410370518.
Abe K, Aoki M, Ikeda M, Watanabe M, Hirai S, Itoyama Y. Clinical characteristics of familial amyotrophic lateral sclerosis with Cu/Zn superoxide dismutase gene mutations. J Neurol Sci. 1996;136(1-2):108–16.
Rouleau GA, Clark AW, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien JP, Figlewicz D. SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol. 1996;39(1):128–31. doi:10.1002/ana.410390119.
Ince PG, Tomkins J, Slade JY, Thatcher NM, Shaw PJ. Amyotrophic lateral sclerosis associated with genetic abnormalities in the gene encoding Cu/Zn superoxide dismutase: molecular pathology of five new cases, and comparison with previous reports and 73 sporadic cases of ALS. J Neuropathol Exp Neurol. 1998;57(10):895–904.
Katayama S, Watanabe C, Noda K, Ohishi H, Yamamura Y, Nishisaka T, Inai K, Asayama K, Murayama S, Nakamura S. Numerous conglomerate inclusions in slowly progressive familial amyotrophic lateral sclerosis with posterior column involvement. J Neurol Sci. 1999;171(1):72–7.
Zu JS, Deng HX, Lo TP, Mitsumoto H, Ahmed MS, Hung WY, Cai ZJ, Tainer JA, Siddique T. Exon 5 encoded domain is not required for the toxic function of mutant SOD1 but essential for the dismutase activity: identification and characterization of two new SOD1 mutations associated with familial amyotrophic lateral sclerosis. Neurogenetics. 1997;1(1):65–71.
Andersen PM, Nilsson P, Keranen ML, Forsgren L, Hagglund J, Karlsborg M, Ronnevi LO, Gredal O, Marklund SL. Phenotypic heterogeneity in motor neuron disease patients with CuZn-superoxide dismutase mutations in Scandinavia. Brain. 1997;120(Pt 10):1723–37.
Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14(6):1105–16.
Chang-Hong R, Wada M, Koyama S, Kimura H, Arawaka S, Kawanami T, Kurita K, Kadoya T, Aoki M, Itoyama Y, Kato T. Neuroprotective effect of oxidized galectin-1 in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;194(1):203–11. doi:10.1016/j.expneurol.2005.02.011.
Wang J, Xu G, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol Dis. 2002;10(2):128–38.
Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12(21):2753–64. doi:10.1093/hmg/ddg312.
Tobisawa S, Hozumi Y, Arawaka S, Koyama S, Wada M, Nagai M, Aoki M, Itoyama Y, Goto K, Kato T. Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice. Biochem Biophys Res Commun. 2003;303(2):496–503.
Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18(2):327–38.
Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Wild-type SOD1 overexpression accelerates disease onset of a G85R SOD1 mouse. Hum Mol Genet. 2009;18(9):1642–51. doi:10.1093/hmg/ddp085.
Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1995;92(3):689–93.
Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation. Hum Mol Genet. 2005;14(16):2335–47. doi:10.1093/hmg/ddi236.
Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, Nilsson P, Marklund SL. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127(Pt 1):73–88. doi:10.1093/brain/awh005.
Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr., Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21(23):9246–54.
Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci U S A. 2002;99(3):1604–9. doi:10.1073/pnas.032539299.
Bahadorani S, Mukai ST, Rabie J, Beckman JS, Phillips JP, Hilliker AJ. Expression of zinc-deficient human superoxide dismutase in Drosophila neurons produces a locomotor defect linked to mitochondrial dysfunction. Neurobiol Aging. 2013;34(10):2322–30. doi:10.1016/j.neurobiolaging.2013.03.024.
Li J, Huang KX, Le WD. Establishing a novel C. elegans model to investigate the role of autophagy in amyotrophic lateral sclerosis. Acta Pharmacol Sin. 2013;34(5):644–50. doi:10.1038/aps.2012.190.
Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, Horwich AL. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009;5(1):e1000350. doi:10.1371/journal.pgen.1000350.
Corrado L, Ratti A, Gellera C, Buratti E, Castellotti B, Carlomagno Y, Ticozzi N, Mazzini L, Testa L, Taroni F, Baralle FE, Silani V, D'Alfonso S. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Hum Mutat. 2009;30(4):688–94. doi:10.1002/humu.20950.
Kirby J, Goodall EF, Smith W, Highley JR, Masanzu R, Hartley JA, Hibberd R, Hollinger HC, Wharton SB, Morrison KE, Ince PG, McDermott CJ, Shaw PJ. Broad clinical phenotypes associated with TAR-DNA binding protein (TARDBP) mutations in amyotrophic lateral sclerosis. Neurogenetics. 2010;11(2):217–25. doi:10.1007/s10048-009-0218-9.
Cairns NJ, Perrin RJ, Schmidt RE, Gru A, Green KG, Carter D, Taylor-Reinwald L, Morris JC, Gitcho MA, Baloh RH. TDP-43 proteinopathy in familial motor neurone disease with TARDBP A315T mutation: a case report. In: Neuropathol Appl Neurobiol, vol 36. vol 7. England: 2010. pp 673-679. doi:10.1111/j.1365-2990.2010.01121.x
Tamaoka A, Arai M, Itokawa M, Arai T, Hasegawa M, Tsuchiya K, Takuma H, Tsuji H, Ishii A, Watanabe M, Takahashi Y, Goto J, Tsuji S, Akiyama H. TDP-43 M337V mutation in familial amyotrophic lateral sclerosis in Japan. Intern Med. 2010;49(4):331–4.
Stallings NR, Puttaparthi K, Luther CM, Burns DK, Elliott JL. Progressive motor weakness in transgenic mice expressing human TDP-43. In: Neurobiol Dis, vol 40. vol 2. United States: Elsevier Inc; 2010. pp 404-414. doi:10.1016/j.nbd.2010.06.017
Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2010;107(8):3858–63. doi:10.1073/pnas.0912417107.
Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci U S A. 2010;107(37):16325–30. doi:10.1073/pnas.1003459107.
Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, Lee VM. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121(2):726–38. doi:10.1172/jci44867.
Tian T, Huang C, Tong J, Yang M, Zhou H, Xia XG. TDP-43 potentiates alpha-synuclein toxicity to dopaminergic neurons in transgenic mice. Int J Biol Sci. 2011;7(2):234–43.
Tsai KJ, Yang CH, Fang YH, Cho KH, Chien WL, Wang WT, Wu TW, Lin CP, Fu WM, Shen CK. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med. 2010;207(8):1661–73. doi:10.1084/jem.20092164.
Stribl C, Samara A, Trumbach D, Peis R, Neumann M, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Rathkolb B, Wolf E, Beckers J, Horsch M, Neff F, Kremmer E, Koob S, Reichert AS, Hans W, Rozman J, Klingenspor M, Aichler M, Walch AK, Becker L, Klopstock T, Glasl L, Holter SM, Wurst W, Floss T. Mitochondrial dysfunction and decrease in body weight of a transgenic knock-in mouse model for TDP-43. J Biol Chem. 2014;289(15):10769–84. doi:10.1074/jbc.M113.515940.
Xu YF, Zhang YJ, Lin WL, Cao X, Stetler C, Dickson DW, Lewis J, Petrucelli L. Expression of mutant TDP-43 induces neuronal dysfunction in transgenic mice. Mol Neurodegener. 2011;6:73. doi:10.1186/1750-1326-6-73.
Janssens J, Wils H, Kleinberger G, Joris G, Cuijt I, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. Overexpression of ALS-associated p.M337V human TDP-43 in mice worsens disease features compared to wild-type human TDP-43 mice. Mol Neurobiol. 2013;48(1):22–35. doi:10.1007/s12035-013-8427-5.
Tong J, Huang C, Bi F, Wu Q, Huang B, Liu X, Li F, Zhou H, Xia XG. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. Embo j. 2013;32(13):1917–26. doi:10.1038/emboj.2013.122.
Li Y, Ray P, Rao EJ, Shi C, Guo W, Chen X, Woodruff EA, 3rd, Fushimi K, Wu JY. A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci U S A. 2010;107(7):3169–74. doi:10.1073/pnas.0913602107.
Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069–75. doi:10.1038/nature09320.
Acknowledgements
We want to thanks Louis-Charles Beland for his grateful help with figures production.
Funding
V.P-M. is a MD-PhD student who holds a Frederick Banting and Charles Best doctoral scholarship from Canadian Institutes of Health Research (CIHR). He is supervised by J-P.J. PhD. who holds a CIHR Canada Research Chair in neurodegeneration and by N.D. MD-MSc. also funded by CIHR and by Canadian Consortium on Neurodegeneration in Aging (CCNA).
Availability of data and materials
Not applicable.
Authors’ contributions
VP-M performed the review of the literature, wrote the paper and prepared figures and tables. PNV PhD. helped with manuscript writing and review of the literature. PVG provides neuropathological pictures of human cases. ND and J-PJ reviewed the paper and supervised the project. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Procedure on human tissue was approved by Laval University ethical committee.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Picher-Martel, V., Valdmanis, P.N., Gould, P.V. et al. From animal models to human disease: a genetic approach for personalized medicine in ALS. acta neuropathol commun 4, 70 (2016). https://doi.org/10.1186/s40478-016-0340-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-016-0340-5