Abstract
Background
Fibrosis is a response to chronic liver disease that results in excessive accumulation of extracellular matrix proteins and formation of scar tissue. Fibrosis represents a clinical challenge of worldwide significance. Several studies have demonstrated that many natural products and herbal medicines have activity against liver fibrosis, and extracts of milk thistle such as silymarin and silybin are the natural compounds most commonly prescribed for liver diseases. Therefore, we sought to assess and compare the pharmacokinetic properties and bioavailability of silybin–phosphatidylcholine complex in oily-medium soft-gel capsules and conventional silymarin tablets in healthy Mexican volunteers.
Methods
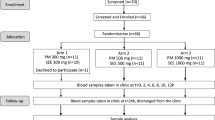
We enrolled 23 healthy volunteers to participate in a prospective, balanced, blind, single-dose, two-way crossover study with a one-week washout period. Fasting participants received either 45 mg silybin–phosphatidylcholine complex or 70 mg silymarin to assess which formulation provided better bioavailability of silybin. Plasma was obtained and analysed for silybin concentration using a validated ultra-performance liquid chromatography–tandem mass spectroscopy method. Pharmacokinetic parameters were obtained by non-compartmental analysis and values were compared by analysis of variance for a crossover design. Ratios of maximum plasma drug concentration and area under the curve (AUC) were obtained and 90% confidence intervals were calculated.
Results
The 23 healthy subjects (11 women, 12 men) who participated in the study were aged 22–31 years old (average: 28), average weight 64.8 kg, height 1.65 m and body mass index 23.5 kg/m2. Plasma levels of silybin were higher after the administration of silybin–phosphatidylcholine complex capsules compared with that after conventional silymarin tablets (P < 0.0001).
Conclusions
The silybin–phosphatidylcholine complex in oily-medium soft-gel capsules seems to provide superior bioavailability. However, clinical studies must be performed to demonstrate its clinical relevance in the treatment of liver diseases.
Trial registration
NCT03440164; registered on November 11, 2016.
Similar content being viewed by others
Background
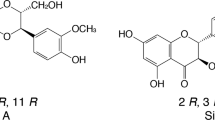
Silymarin (SM) is a complex of one flavonoid (taxifolin) and at least seven flavonolignans, which are the most common class of substance present in milk thistle (Silybum marianum) extract [1]. These flavonolignans include silibinin, isosilibinin, silychristin, isosilychristin and silydianin [2], of which silybin (SIB) is the most prevalent substance with the most important biological activity. The relative abundance of SIB in SM can differ depending on the source of the botanical material, the provider and the extraction method [3]. However, SIB represents approximately 50 to 70% of the total composition of SM extract.
SM has been used for over 2000 years as a single-herb compound for treating liver diseases such as hepatitis, cirrhosis and jaundice, and as a remedy against poisoning from chemical and environmental toxins [1]. It has been demonstrated that SM protects against liver injury by means of its antioxidant, anti-inflammatory, anti-fibrotic, metabolic and cellular signalling effects [4,5,6]. In terms of cell signalling, SIB has been considered a chemopreventive and cancer-protective drug because of its effect on mitogenic signalling and cell-cycle regulation through induction of apoptosis and the inhibition of growth factor receptor-mediated mitogenic and cell survival signalling [7]. In vivo, SM and SIB have been used to manage alcoholic liver disease, non-alcoholic liver disease, liver fibrosis and cirrhosis [8]. Inhabitants in several European countries still use SIB to treat a range of hepatobiliary problems [9].
It is known that flavonolignans have poor and irregular bioavailability. The absorption rate of SM varies between 20 and 50% [10], while SIB has shown a straight-line concentration–response relationship over a concentration range of 0.5–100 μg/mL [11]. Many soluble derivatives of SIB have been synthesised to counteract its low solubility, bioavailability and absorption, including silybin bis-hemisuccinate, β-cyclodextrin complex, silybin-N-methyl-glucamine, silybin-11-O-phosphate and silybin-phosphatidylcholine [12,13,14]. In animal studies, silybin–phosphatidylcholine complex (SPC) has been shown to reduce oxidative stress, lipid peroxidation and collagen accumulation and, as a consequence, reduce liver damage [15]. Further, enzymatic synthesis of SIB β-glycosides such as silybin β-galactoside, silybin β-glucoside, silybin β-maltoside and silybin β-lactoside, have shown that they have improved SIB solubility [16]. Many studies have demonstrated in several chronic liver diseases that SIB and SM are safe and well-tolerated compounds with a limited adverse event profile [1, 5, 17]. Therefore, we sought to assess whether the bioavailability of SIB is increased by the administration of SPC in oily-medium soft-gel capsules compared with plasma levels of SIB achieved after the administration of conventional SM tablets.
Methods
Clinical protocol
The study began with 24 healthy volunteers. One volunteer dropped out because he took a non-authorized medication during the washout period. Twenty-three volunteers completed the clinical study. The volunteers of both sexes (12 men and 11 women) selected for the study were between 18 and 44 years old with body mass index ≥18 and ≤ 27. All subjects provided informed consent, and the Ethics Committee of Núcleo Clínico de Bioequivalencia, SA de CV (NABIO) and the Federal Commission for the Protection against Sanitary Risk (COFEPRIS) approved the clinical protocol. The study was performed in accordance with the principles of the Declaration of Helsinki (2013), General Health Law in Mexico and ICH-Good Clinical Practice (2005). All volunteers were healthy as assessed by electrocardiography, physical examination and the following laboratory tests: routine urinalysis, fasting blood glucose, total cholesterol, triglycerides, albumin, urea, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase and total bilirubin and its fractions. All subjects were negative for human immunodeficiency virus, hepatitis B virus and hepatitis C virus (HCV). It was also verified that participants were free from significant cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal and haematological diseases.
The study was conducted as a prospective, single-blind, randomized, two-period crossover balanced design with a one-week washout period between the doses. During each period, the volunteers were hospitalized 13 h before being given the drug. A xanthine-free meal was given 11 h before administering the drug. After a 10-h fasting period, they received a 45 mg dose of SPC in oily-medium soft-gel capsules or a 70 mg tablet of conventional SM. The drugs were given at 7:00 h directly into the volunteer’s mouth, followed by 250 mL of tap water. All volunteers fasted for 4 h after drug administration, when a xanthine-free standard breakfast was consumed. A standard meal was provided 8 h after dosing. No other meal was allowed during the “in-house” period. Systolic and diastolic arterial blood pressure (measured with a sphygmomanometer) and heart rate were recorded before and after drug administration.
The methodology used was similar to that previously described in other studies on bioavailability of different drugs.
Formulations
The following test formulations were employed: SPC in oily-medium soft-gel capsules 45 mg (NeoCholal-S®, batch number 601031, expiration date 01/2018) made by Laboratorios Italmex S.A., Mexico City (Mexico) and SM tablets 70 mg (Legalon®, batch number 11149075, expiration date 05/2019) made by Laboratorios Takeda S.A., Mexico City (Mexico).
Drug analysis
Blood samples (8 mL) from a suitable forearm vein were collected into heparin-containing tubes before drug administration (0) as well as at 0.16, 0.33, 0.50, 0.66, 0.83, 1, 1.25, 1.50, 2, 2.50, 3, 3.50, 4, 5, 6 and 8 h post dosing with each formulation. The blood samples were centrifuged at 4000 rpm for 15 min at 6 °C. Plasma was transferred to labelled tubes and stored at − 80 ± 20 °C until analysis. For drug analysis, 0.75 mL of methanol was added to a conical glass tube, followed by the plasma sample (0.5 mL). The tube was vortex-mixed for 90 s and then centrifuged at 4000 rpm for 15 min at 6 °C. The organic extracts were filtered through a 0.2-μm pore-size nylon Acrodisc. Aliquots (5 μL) were analysed by an ultra-performance liquid chromatography–tandem mass spectrometry model Acquity UPLC brand waters from Boston, USA. This model it was used to measure the concentration of SIB. The mobile phase consisted of 0.1% formic acid and methanol (20:80) at a constant flow rate (0.15 mL/min). One week after the first dose (i.e., after the washout period), the same procedure was repeated with the alternate drug.
Calibration standards and quality control
The calibration curve was reproducible and linear based on the method used. Standard solutions were prepared from the stock solution by sequential dilutions to give eight concentrations: 1, 5, 10, 25, 50, 75, 100 and 150 ng/mL, as well as quality control (QC) plasma samples with concentrations of 3 ng/mL (low), 30 ng/mL (medium) and 120 ng/mL (high). Calibration standards were generated by spiking control human plasma with the respective standard solutions. The calibration standards and blanks were prepared in duplicate for each assay and were processed together with plasma samples and low (QCA), medium (QCB) and high (QCC) quality-control samples.
Tolerability
Tolerability was assessed by monitoring changes from baseline in vital signs (blood pressure, heart rate, temperature and respiratory rate). Laboratory tests (haematology, biochemistry, liver function and urinalysis) were performed before dosing in each period and at the completion of the study. A clinician questioned subjects about adverse events occurring during the study or washout period, and recorded adverse events on an appropriate form.
Pharmacokinetics and statistical analysis
Pharmacokinetic and statistical analyses were performed using the non-compartmental analysis in Phoenix WinNolin software (version 6.1; Pharsight Corporation, Mountain View, CA, USA). Maximum plasma drug concentration (Cmax), the area under the plasma concentration-time curve from time 0 to the last sampling time (AUC0–t), the area under the plasma concentration-time curve from time 0 to infinity (AUC0–∞), the time to reach Cmax (Tmax), the half-life (t1/2) and the first-order terminal elimination rate constant (ke) were determined for each subject. Ke was obtained from the slope of the linear regression of the log-transformed concentration–time curve in the terminal phase. Cmax and Tmax were obtained directly from the curves. The areas under the SIB plasma concentration vs. time curves from 0 to 8 h (AUClast) were calculated by applying the linear trapezoid rule. The AUC0–∞ was determined by adding the value Clast/ke to the calculated AUClast (where Clast means the last detectable concentration). Pharmacokinetic parameters were compared using analysis of variance for a cross-over design to evaluate the bioequivalence of the test and reference formulations. Calculation of 90% confidence intervals for the ratio of test to reference treatment was conducted for Cmax and AUC0–∞.
Results
The content of SIB in the conventional tablets was 29.5 mg while that in the SPC capsules was 47.1 mg. The mean SIB plasma concentrations of the 23 volunteers after a 45 mg oral dose of SPC complex in oily-medium soft-gel capsules or 70 mg of conventional SM tablets are shown in Fig. 1. The respective mean pharmacokinetic parameters are shown in Table 1. SIB peak plasma concentrations were 207.1 ng/L for SPC and 12.6 ng/L for SM tablets. All pharmacokinetic parameters differed significantly between formulations (P < 0.0001). Both formulations were well tolerated with no serious adverse events observed. However, two adverse events were reported: mild epigastric pain and mild headache. The first one was related to conventional SM tablets and the second one was related to silybin-phosphatidylcholine complex.
Discussion
Many pharmacokinetic studies have shown that silybin-phosphatidylcholine improves the bioavailability of SIB in both healthy humans and in people with chronic liver disease. The present study demonstrated that SIB bioavailability is 9.6 times higher with SPC in oily-medium soft-gel capsules compared with conventional SM tablets. Several studies conducted in animals or humans have demonstrated that the complex of silybin with phosphatidylcholine has superior bioavailability over non-complexed SIB [3, 4, 18,19,20]. However, our results show the highest biological availability of SPC of all currently available studies.
There is still controversy about the effects of SIB on liver damage [21] because of the lack of definitive clinical data about the efficacy of SM or any of the current SIB preparations in the treatment of chronic liver disease. Some limitations of clinical studies could be related to lack of budget, small sample size, or lack of information about the type and dose of extract used and product classification [22,23,24]. Nevertheless, the absence of severe adverse events associated with high doses of SIB is well defined [25].
It is well known that oxidative and nitrosative stress increase the accumulation of extracellular matrix, which plays a crucial role in liver fibrosis [26]. In this context, it has been demonstrated that SPC at a dose ranging from 240 mg/d to 942 mg t.i.d. (the highest dose used in patients with liver damage) can significantly improve oxidative stress by decreasing malondialdehyde levels [3]. Therefore, this complex could be a useful antioxidant-based chemopreventive therapy to balance cellular redox. Further, some studies have evaluated the pharmacokinetics of SIB in patients with non-alcoholic fatty liver disease (NAFLD) [27, 28].
It has been reported that SM or any SIB formulation can induce a meaningful decrease in markers of chronic inflammation, metabolic parameters, degree of liver steatosis and fibrosis and an improvement in liver function tests [29, 30]. SIB has been shown to prevent mitochondrial dysfunction in animal models and to reduce liver damage in NAFLD patients [31]. In addition, similar outcomes have been reported for the use of these compounds in patients with HCV [32,33,34,35,36,37]. Consequently, SIB could be an alternative or complementary therapeutic option, particularly, when other drugs are not indicated or have failed.
Conclusions
The presently available data demonstrates that SM or SIB formulations can be useful to treat several liver diseases. There is a need for drugs (especially for treating chronic liver disease) that can be used long-term without serious adverse events. SM and SIB formulations seem to meet this goal. This study clearly shows that SIB has superior bioavailability in healthy volunteers when administered in SPC in oily-medium soft-gel capsules compared with conventional SM tablets. However, more clinical studies must be performed to demonstrate the clinical relevance of these results for treatment of liver disease.
Change history
22 February 2019
Abstract
Following publication of the original article [1], the author reported their given name have been erroneously tagged as their family names.
25 June 2021
A Correction to this paper has been published: https://doi.org/10.1186/s40360-021-00500-2
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ECG:
-
Electrocardiography
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- NAFLD:
-
Non-Alcoholic Fatty Liver Disease
- SIB:
-
Silybin
- SM:
-
Silymarin
- SPC:
-
Silybin–Phosphatidylcholine Complex
- γGT:
-
γ-Glutamyl transferase
References
Gazák R, Walterová D, Kren V. Silybin and silymarin—new and emerging applications in medicine. Curr Med Chem. 2007;14(3):315–38.
Simánek V, Kren V, Ulrichová J, Vicar J, Cvak L. Silymarin: what is in the name … ? An appeal for a change of editorial policy. Hepatology. 2000;32(2):442–4.
Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol. 2011;17(18):2288–301.
Barzaghi N, Crema F, Gatti G, Pifferi G, Perruca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet. 1990;15(4):333–8.
Zarrelli A, Romanucci V, Tuccillo C, Federico A, Loguercio C, Gravante R, et al. New silibinin glyco-conjugates: synthesis and evaluation of antioxidant properties. Bioorg Med Chem Lett. 2014;24(22):5147–9.
Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Ann Hepatol. 2017;16(1):48–56.
Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, et al. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: relationship with silibinin levels. Eur J Cancer. 2008;44(6):898–906.
Lirussi F, Beccarello A, Zanette G, DeMonte A, Donadon V, Velussi M, et al. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab. 2002;15(4):222–31.
Pradeep K, Chandrasekaran V, Gobianand K, Karthikeyan S. Silymarin: an effective hepatoprotective agent against diethylnitrosamine-induced hepatotoxicity in rats. Pharm Biol. 2007;45(9):707–14.
Kvasnicka F, Bíba B, Sevcík R, Voldrich M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990(1–2):239–45.
Lee JI, Narayan M, Barrett JS. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;845(1):95–103.
Dunnick JK, Singh B, Nyska A, Peckham J, Kissling GE, Sanders JM. Investigating the potential for toxicity from long-term use of the herbal products, goldenseal and milk thistle. Toxicol Pathol. 2011;39(2):398–409.
Saller R, Brignoli R, Melzer MR. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15(1):9–20.
Lieber C, Robins S, Li J, DeCarli LM, Mak KM, Fasulo JM, Leo MA. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology. 1994;106(1):152–9.
Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 2006;30(1):77–82.
Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16(3):239–49.
Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R. Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. 2018.
Kidd P. Head K. a review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos). Altern Med Rev. 2005;10(3):193–203.
Filburn CR, Kettenacker R, Griffin DW. Bioavailability of a silybin-phosphatidylcholine complex in dogs. J Vet Pharmacol Ther. 2007;30(2):132–8.
Webb CB, Gustafson DL, Twedt D. Bioavailability following oral administration of a silibinin-phosphatidylcholine complex in cats. Intern J Appl Res Vet Med. 2010;10(2):107–12.
Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–63.
Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther. 2007;6(2):146–57.
Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39(4):293–304.
Fehér J, Lengyel G. Silymarin in the treatment of chronic liver diseases: past and future. Orv Hetil. 2008;149(51):2413–8.
Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, Esquivel-Soto J, Esquivel-Chirino C, Garcia-Luna y Gonzalez-Rubio M, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144–9.
Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19(28):4850–60.
Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules. 2017;22(2):E191.
Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113(6):506–15.
Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, et al. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. 2006;55(6):901–2.
Federico A, Niosi M, Vecchio Blanco CD, Loguercio C. Emerging drugs for non-alcoholic fatty liver disease. Expert Opin Emerg Drugs. 2008;13(1):145–58.
Serviddio G, Bellanti F, Giudetti AM, Gnoni GV, Petrella A, Tamborra R, et al. A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2010;332(3):922–32.
Chan WK, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940–9.
Aller R, Izaola O, Gómez S, Tafur C, Gonzalez G, Berroa E, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19(16):3118–24.
Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135(5):1561–7.
Biermer M, Berg T. Rapid suppression of hepatitis C viremia induced by intravenous silibinin plus ribavirin. Gastroenterology. 2009;137(1):390–1.
Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, et al. HALT-C trial group. Silymarin use and liver disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Aliment Pharmacol Ther. 2011;33(1):127–37.
Ahmed-Belkacem A, Ahnou N, Barbotte L, Wichoswky C, Pallier C, Brillet R, et al. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology. 2010;138(3):1112–22.
Acknowledgments
The authors would like to thank Italmex Pharma S.A. and Medica Sur Clinic & Foundation.
Funding
This study was supported partially by ITALMEX SA providing professional laboratory services, material for test and assistance for the methodology in the performance of biochemical process.
Availability of data and materials
The datasets used and/or analysed for the current study are available from the corresponding author on reasonable request.
Author contributions
Confirmed, that all authors read and approved the final manuscript. NMS-Principal investigator. NMS - Designed the study. NMS - Reviewed the literature. NMS- Wrote the manuscript. JSN clinical investigator. JSN - Reviewed the literature. JSN - Collected the data. MDM - Reviewed the literature. MDM- Collected the data. RSM -Reviewed the literature. RSM- Collected the data. FFM-Designed the study. FFM- Analysed data. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics & Investigation Committee of Nucleo Clínico de Bioequivalencia, S.A. de C.V. (NABIO) and the Mexican Health Authority (COFEPRIS). The study was conducted in accordance with the provisions of the Declaration of Helsinki (2013), General Health Law in Mexico and ICH Good Clinical Practice procedures (2005).
The participants granted the written consent to participate in the study. The signed form was approved by NABIO and COFEPRIS.
Consent for publication
Not applicable.
Competing interests
MDM is a member of research group from ITALMEX SA, He was declare no competing interests. JSN – Is a member of research group from ITALMEX SA, He was declare no competing interests. RSM – Is a member of research group from ITALMEX SA, he was declare no competing interests. All other authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
The original version of this article was revised. The authors reported their given name have been erroneously tagged as their family names.The correct names are: Given name Nahum Family name Méndez-Sánchez, Given name Miguel Family name Dibildox-Martinez, Given name Jahir Family name Sosa-Noguera Jahir, Given name Ramón Family name Sánchez-Medal, Given name Francisco J. Family name Flores-Murrieta.
an error was identified in the Results section, with regard to values of Table 1. The incorrect sentence is: SIB peak plasma concentrations were 207.1 mg/L for SPC and 12.6 mg/L for SM tablets. All pharmacokinetic parameters differed significantly between formulations (P < 0.0001). The correct sentence is: SIB peak plasma concentrations were 207.1 ng/L for SPC and 12.6 ng/L for SM tablets. All pharmacokinetic parameters differed significantly between formulations (P < 0.0001).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Méndez-Sánchez, N., Dibildox-Martinez, M., Sosa-Noguera, J. et al. Superior silybin bioavailability of silybin–phosphatidylcholine complex in oily-medium soft-gel capsules versus conventional silymarin tablets in healthy volunteers*. BMC Pharmacol Toxicol 20, 5 (2019). https://doi.org/10.1186/s40360-018-0280-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-018-0280-8